
A capillary is a small blood vessel, from 5 to 10 micrometres in diameter, and is part of the microcirculation system. Capillaries are microvessels and the smallest blood vessels in the body. They are composed of only the tunica intima, consisting of a thin wall of simple squamous endothelial cells. They are the site of the exchange of many substances from the surrounding interstitial fluid, and they convey blood from the smallest branches of the arteries (arterioles) to those of the veins (venules). Other substances which cross capillaries include water, oxygen, carbon dioxide, urea, glucose, uric acid, lactic acid and creatinine. Lymph capillaries connect with larger lymph vessels to drain lymphatic fluid collected in microcirculation.
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function.

The microcirculation is the circulation of the blood in the smallest blood vessels, the microvessels of the microvasculature present within organ tissues. The microvessels include terminal arterioles, metarterioles, capillaries, and venules. Arterioles carry oxygenated blood to the capillaries, and blood flows out of the capillaries through venules into veins.

The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells form the barrier between vessels and tissue and control the flow of substances and fluid into and out of a tissue.
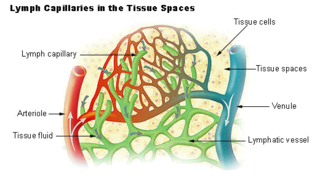
The lymphatic vessels are thin-walled vessels (tubes), structured like blood vessels, that carry lymph. As part of the lymphatic system, lymph vessels are complementary to the cardiovascular system. Lymph vessels are lined by endothelial cells, and have a thin layer of smooth muscle, and adventitia that binds the lymph vessels to the surrounding tissue. Lymph vessels are devoted to the propulsion of the lymph from the lymph capillaries, which are mainly concerned with the absorption of interstitial fluid from the tissues. Lymph capillaries are slightly bigger than their counterpart capillaries of the vascular system. Lymph vessels that carry lymph to a lymph node are called afferent lymph vessels, and those that carry it from a lymph node are called efferent lymph vessels, from where the lymph may travel to another lymph node, may be returned to a vein, or may travel to a larger lymph duct. Lymph ducts drain the lymph into one of the subclavian veins and thus return it to general circulation.
Mesangial cells are specialised cells in the kidney that make up the mesangium of the glomerulus. Together with the mesangial matrix, they form the vascular pole of the renal corpuscle. The mesangial cell population accounts for approximately 30-40% of the total cells in the glomerulus. Mesangial cells can be categorized as either extraglomerular mesangial cells or intraglomerular mesangial cells, based on their relative location to the glomerulus. The extraglomerular mesangial cells are found between the afferent and efferent arterioles towards the vascular pole of the glomerulus. The extraglomerular mesangial cells are adjacent to the intraglomerular mesangial cells that are located inside the glomerulus and in between the capillaries. The primary function of mesangial cells is to remove trapped residues and aggregated protein from the basement membrane thus keeping the filter free of debris. The contractile properties of mesangial cells have been shown to be insignificant in changing the filtration pressure of the glomerulus.

The glycocalyx, also known as the pericellular matrix and sometime cell coat, is a glycoprotein and glycolipid covering that surrounds the cell membranes of bacteria, epithelial cells, and other cells. It was described in a review article in 1970.
The Starling principle holds that extracellular fluid movements between blood and tissues are determined by differences in hydrostatic pressure and colloid osmotic (oncotic) pressure between plasma inside microvessels and interstitial fluid outside them. The Starling Equation, proposed many years after the death of Starling, describes that relationship in mathematical form and can be applied to many biological and non-biological semipermeable membranes. The classic Starling principle and the equation that describes it have in recent years been revised and extended.

Pericytes are multi-functional mural cells of the microcirculation that wrap around the endothelial cells that line the capillaries throughout the body. Pericytes are embedded in the basement membrane of blood capillaries, where they communicate with endothelial cells by means of both direct physical contact and paracrine signaling. The morphology, distribution, density and molecular fingerprints of pericytes vary between organs and vascular beds. Pericytes help to maintain homeostatic and hemostatic functions in the brain, one of the organs with higher pericyte coverage, and also sustain the blood–brain barrier. These cells are also a key component of the neurovascular unit, which includes endothelial cells, astrocytes, and neurons. Pericytes have been postulated to regulate capillary blood flow and the clearance and phagocytosis of cellular debris in vitro. Pericytes stabilize and monitor the maturation of endothelial cells by means of direct communication between the cell membrane as well as through paracrine signaling. A deficiency of pericytes in the central nervous system can cause increased permeability of the blood–brain barrier.

Hyperaemia is the increase of blood flow to different tissues in the body. It can have medical implications but is also a regulatory response, allowing change in blood supply to different tissues through vasodilation. Clinically, hyperaemia in tissues manifests as erythema because of the engorgement of vessels with oxygenated blood. Hyperaemia can also occur due to a fall in atmospheric pressure outside the body. The term is from Greek ὑπέρ (hupér) 'over' + αἷμα (haîma) 'blood'.
Neovascularization is the natural formation of new blood vessels, usually in the form of functional microvascular networks, capable of perfusion by red blood cells, that form to serve as collateral circulation in response to local poor perfusion or ischemia.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Vascular endothelial growth factor A (VEGF-A) is a protein that in humans is encoded by the VEGFA gene.

Vascular remodelling is a process which occurs when an immature heart begins contracting, pushing fluid through the early vasculature. The process typically begins at day 22, and continues to the tenth week of human embryogenesis. This first passage of fluid initiates a signal cascade and cell movement based on physical cues including shear stress and circumferential stress, which is necessary for the remodelling of the vascular network, arterial-venous identity, angiogenesis, and the regulation of genes through mechanotransduction. This embryonic process is necessary for the future stability of the mature vascular network.
Microvasculature comprises the microvessels – venules and capillaries of the microcirculation, with a maximum average diameter of 0.3 millimeters. As the vessels decrease in size, they increase their surface-area-to-volume ratio. This allows surface properties to play a significant role in the function of the vessel.
Neuroangiogenesis is the coordinated growth of nerves and blood vessels. The nervous and blood vessel systems share guidance cues and cell-surface receptors allowing for this synchronised growth. The term neuroangiogenesis only came into use in 2002 and the process was previously known as neurovascular patterning. The combination of neurogenesis and angiogenesis is an essential part of embryonic development and early life. It is thought to have a role in pathologies such as endometriosis, brain tumors, and Alzheimer's disease.

Tumor-associated endothelial cells or tumor endothelial cells (TECs) refers to cells lining the tumor-associated blood vessels that control the passage of nutrients into surrounding tumor tissue. Across different cancer types, tumor-associated blood vessels have been discovered to differ significantly from normal blood vessels in morphology, gene expression, and functionality in ways that promote cancer progression. There has been notable interest in developing cancer therapeutics that capitalize on these abnormalities of the tumor-associated endothelium to destroy tumors.
The neurovascular unit (NVU) comprises the components of the brain that collectively regulate cerebral blood flow in order to deliver the requisite nutrients to activated neurons. The NVU addresses the brain's unique dilemma of having high energy demands yet low energy storage capacity. In order to function properly, the brain must receive substrates for energy metabolism–mainly glucose–in specific areas, quantities, and times. Neurons do not have the same ability as, for example, muscle cells, which can use up their energy reserves and refill them later; therefore, cerebral metabolism must be driven in the moment. The neurovascular unit facilitates this ad hoc delivery and, thus, ensures that neuronal activity can continue seamlessly.
VINE-seq is a method to isolate and molecularly characterize the vascular and perivascular cells of the human brain microvessels at single-nuclei resolution. This technique is achieved by combining various known laboratory-based strategies involving the mechanical dissociation of brain tissue samples into single cells, density gradient centrifugation and filtration to isolate nuclei of microvessels, fluorescence-activated cell sorting (FACs) of cellular populations and droplet-based single-nuclei RNA sequencing (drop-snRNA-seq). Altogether, this generates a single-nuclei transcriptomic profile of the various cell types present in the vasculature of the brain. Through processing and analyzing the single-nuclei transcriptomic data, the heterogeneity within and between cell types can be distinguished to construct the molecular landscape of the human brain vasculature that was not previously done before.













