Structure
Vejocalcin has a molecular mass of approximately 3.8 kDa and an isoelectric point of 9.3. [1]
Physical and chemical characteristics of vejocalcin [1] | Formula | Amino Acids | Molecular Mass | Molecular Volume | Negatively charged residues | Positively charged residues |
|---|
| C149H254N56O47S6 | 33 | 3,774.4 | 2,692.7 | 3 (9%) | 9 (27%) |
It is a relatively small protein, consisting of only 33 amino acids:
Ala-Asp-Cys-Leu-Ala-His-Leu-Lys-Leu-Cys-Lys-Lys-Asn-Asn-Asp-Cys-Cys-Ser-Lys-Lys-Cys-Ser-Arg-Arg-Gly-Thr-Asn-Pro-Glu-Glu-Arg-Cys-Arg
Notably, two calcins produced by two closely related scorpions - vejocalcin from Vaejovis mexicanus and intrepicalcin from Vaejovis intrepidus - display a 97% similarity in their primary sequence, differing in only one amino acid at position 14 (Asn and Lys, respectively). Despite this marked similarity, vejocalcin exhibits a binding affinity to RyR1 that is 4.7-fold higher than that of intrepicalcin. [1]
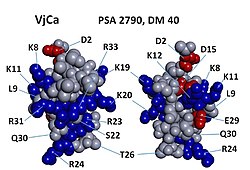
Vejocalcin shows an arrangement of charged residues, in which most of the positively charged residues are segregated on one side of the molecule, whereas neutral and negatively charged residues are clustered on the opposite side. [1] This arrangement generates a discrete dipole moment (DM) and appears to be a prevalent feature across all toxins of the calcin family. [4] Interestingly, vejocalcin has the smallest charge segregation among peptides in the calcin family. However, comparisons among different calcins show that, for each peptide, there appears to be no correlation between DM, binding affinity and subconductance state attributes. [1] [5]
Maturation of vejocalcin involves post-translational modification of its tertiary structure. Specifically, three disulfide bonds are formed between cysteine residues in positions 3–17, 10–21, and 16–32. [1] These three disulfide bonds arrange themselves spatially to form a “disulfide through disulfide knot”, which is an evolutionary conserved structural motif known as the inhibitor cystine knot motif (ICK motif), thus defining the whole protein as a knottin. [6] This three-dimensional arrangement confers the protein remarkable stability and builds the structural core of its pharmacological active site. ICK motifs have also been shown to be characteristic of calcium channel blocking toxins produced by snails and spiders. [1]