
A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration.

The halogens are a group in the periodic table consisting of five chemically related elements: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At). The artificially created element 117 may also be a halogen. In the modern IUPAC nomenclature, this group is known as group 17. The symbol X is often used generically to refer to any halogen.

A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group, particularly the second-row elements nitrogen (N), oxygen (O), or fluorine (F)—the hydrogen bond donor (Dn)—and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn–H···Ac, where the solid line denotes a fully covalent bond, and the dotted line indicates the hydrogen bond. There is general agreement that there is actually a minor covalent component to hydrogen bonding, especially for moderate to strong hydrogen bonds, although the importance of covalency in hydrogen bonding is debated. At the opposite end of the scale, there is no clear boundary between a weak hydrogen bond and a van der Waals interaction. Weaker hydrogen bonds are known for hydrogen atoms bound to elements such as sulfur (S) or chlorine (Cl); even carbon (C) can serve as a donor, particularly when the carbon or one of its neighbors is electronegative. The hydrogen bond is responsible for many of the anomalous physical and chemical properties of compounds of N, O, and F.
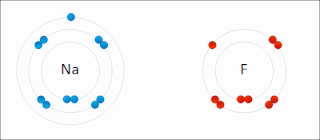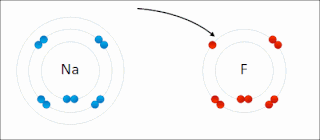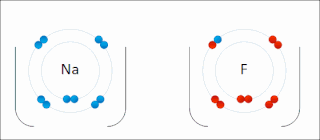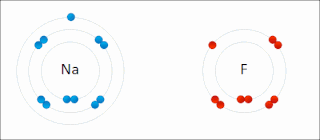
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. It is one of the main bonds along with Covalent bond and Metallic bonding. Ions are atoms that have gained one or more electrons and atoms that have lost one or more electrons. This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH+
4 or SO2−
4. In simpler words, an ionic bond is the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms.

A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.

Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (kL) and the heavy (kH) isotopically substituted reactants (isotopologues):
An interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
The bond-dissociation energy (BDE, D0, or DH°) is one measure of the strength of a chemical bond A–B. It can be defined as the standard enthalpy change when A–B is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K (standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C–H bonds in ethane (C2H6) is defined as the standard enthalpy change of the process
In chemistry, bond energy (E) or bond enthalpy (H) is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond dissociation energies (usually at a temperature of 298 K) for all bonds of the same type within the same chemical species. For example, the carbon–hydrogen bond energy in methane H(C–H) is the enthalpy change involved with breaking up one molecule of methane into a carbon atom and four hydrogen radicals, divided by 4. Tabulated bond energies are generally values of bond energies averaged over a number of selected typical chemical species containing that type of bond. Bond energy (E) or bond enthalpy (H) should not be confused with bond-dissociation energy. Bond energy is the average of all the bond-dissociation energies in a molecule, and will show a different value for a given bond than the bond-dissociation energy would. This is because the energy required to break a single bond in a specific molecule differs for each bond in that molecule. For example, methane has four C–H bonds and the bond-dissociation energies are 435 kJ/mol for D(CH3–H), 444 kJ/mol for D(CH2–H), 444 kJ/mol for D(CH–H) and 339 kJ/mol for D(C–H). Their average, and hence the bond energy, is 414 kJ/mol, even though not a single bond required specifically 414 kJ/mol to be broken.
In chemistry, heterolysis or heterolytic fission is the process of cleaving a covalent bond where one previously bonded species takes both original bonding electrons from the other species. During heterolytic bond cleavage of a neutral molecule, a cation and an anion will be generated. Most commonly the more electronegative atom keeps the pair of electrons becoming anionic while the more electropositive atom becomes cationic.
A non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1-5 kcal/mol. Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.

A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces, dipole-dipole interactions, quadrupole interactions, π-π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic interactions. Van der Waals, dipole interactions, quadrupole interactions, π-π interactions, hydrogen bonding, and halogen bonding are typically much weaker than the forces holding together other solids: metallic, ionic, and network solids. Intermolecular interactions, typically do not involve delocalized electrons, unlike metallic and certain covalent bonds. Exceptions are charge-transfer complexes such as the tetrathiafulvane-tetracyanoquinodimethane (TTF-TCNQ), a radical ion salt. These differences in the strength of force and electronic characteristics from other types of solids give rise to the unique mechanical, electronic, and thermal properties of molecular solids.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it has an extensive vocabulary and a significant amount of jargon.

A chemical compound is a chemical substance composed of many identical molecules composed of atoms from more than one element held together by chemical bonds. A chemical element bonded to an identical chemical element is not a chemical compound since only one element, not two different elements, is involved.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.















