
β-Carboline (9H-pyrido[3,4-b]indole), also known as norharmane, is a nitrogen containing heterocycle. It is also the prototype of a class of indole alkaloid compounds known as β-carbolines.
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler. Traditionally an acidic catalyst in protic solvent was employed with heating, however the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as Kratom. It is often referred to as ‘7-OH’. It was first described in 1994 and is a natural product derived from the mitragynine present in the Kratom leaf. It is considered an oxidized derivative and active metabolite of mitragynine.

Alstonia scholaris, commonly called blackboard tree or devil's tree in English, is an evergreen tropical tree in the family Apocynaceae. It is native to southern China, tropical Asia and Australasia, it is a commonly planted ornamental plant in these areas. It is a toxic plant, but traditionally it is used medicinally for myriad diseases and complaints.

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
Strictosidine synthase (EC 4.3.3.2) a key enzyme in alkaloid biosynthesis. It catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

In enzymology, a polyneuridine-aldehyde esterase (EC 3.1.1.78) is an enzyme that catalyzes the chemical reaction:
In enzymology, a vinorine synthase is an enzyme that catalyzes the chemical reaction

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.
The molecular formula C21H22N2O2 may refer to:

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
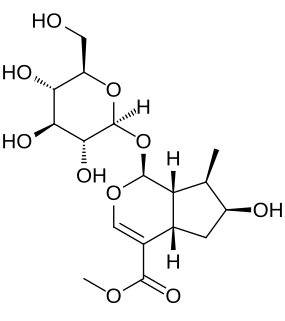
Loganin is one of the best-known of the iridoid glycosides. It is named for the Loganiaceae, having first been isolated from the seeds of a member of that plant family, namely those of Strychnos nux-vomica. It also occurs in Alstonia boonei (Apocynaceae), a medicinal tree of West Africa and in the medicinal/entheogenic shrub Desfontainia spinosa (Columelliaceae) native to Central America and South America.

Echitamidine is an indole alkaloid isolated from Alstonia boonei. Its laboratory synthesis has been reported.

Alschomine is an indole alkaloid first identified in the leaves of Alstonia scholaris in 1989.
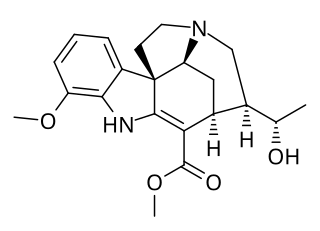
Scholarine is an alkaloid isolated initially from Alstonia scholaris by Banerji and Siddhanta. Subsequently, it was obtained from the West African tree Alstonia boonei. The derivative N-Formylscholarine has been isolated from the fruit pods of Alstonia scholaris.
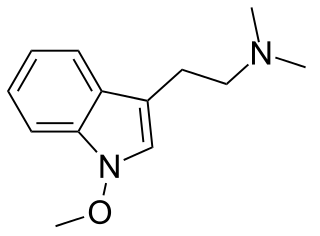
Lespedamine is an indole alkaloid and substituted tryptamine present in the plant Lespedeza bicolor. The alkaloid bears a close structural resemblance to the psychedelic alkaloid dimethyltryptamine and was speculated to have psychoactivity by Alexander Shulgin. No reports on lespedamine's biological activity have been published.

4,21-Dehydrogeissoschizine is a terpene indole alkaloid. It is believed to be the precursor leading to the formation of the aspidosperma, corynanthe, and iboga classes of terpene indole alkaloids.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.
















