
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.
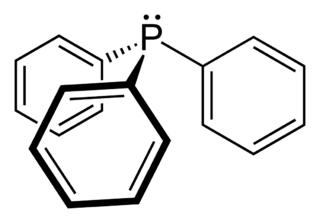
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry.

Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.

Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.

Organozirconium compounds are organometallic compounds containing a carbon to zirconium chemical bond. Organozirconium chemistry is the corresponding science exploring properties, structure, and reactivity of these compounds. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
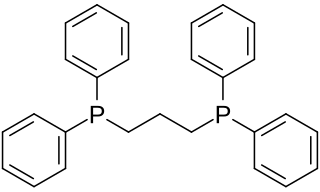
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis.
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. The process is the basis of organosilicon chemistry.

Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.
Organosilver chemistry in chemistry of compounds containing a carbon to silver chemical bond. The theme is less developed than organocopper chemistry.

Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used in homogeneous catalysis.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

A metal-phosphine complex is a In coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in homogeneous catalysis.

















