
Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.
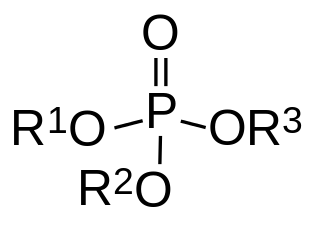
In organic chemistry, organophosphates (also known as phosphate esters, phosphorates(V) or OPEs) are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides.

MCPA is a widely used phenoxy herbicide introduced in 1945. It selectively controls broad-leaf weeds in pasture and cereal crops. The mode of action of MCPA is as an auxin, which are growth hormones that naturally exist in plants.
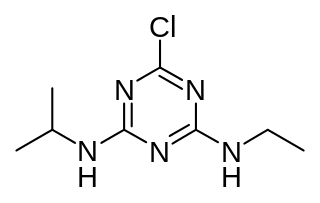
Atrazine is a chlorinated herbicide of the triazine class. It is used to prevent pre-emergence broadleaf weeds in crops such as maize (corn), soybean and sugarcane and on turf, such as golf courses and residential lawns. Atrazine's primary manufacturer is Syngenta and it is one of the most widely used herbicides in the United States, Canadian, and Australian agriculture. Its use was banned in the European Union in 2004, when the EU found groundwater levels exceeding the limits set by regulators, and Syngenta could not show that this could be prevented nor that these levels were safe.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.

Hexazinone is an organic compound that is used as a broad spectrum herbicide. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the triazine class herbicides, it is manufactured by DuPont and sold under the trade name Velpar.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine—the "symmetric" isomer—and its derivatives are useful in a variety of applications.
Pelargonic acid, also called nonanoic acid, is an organic compound with structural formula CH3(CH2)7CO2H. It is a nine-carbon fatty acid. Nonanoic acid is a colorless oily liquid with an unpleasant, rancid odor. It is nearly insoluble in water, but very soluble in organic solvents. The esters and salts of pelargonic acid are called pelargonates or nonanoates.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions. It is a Schedule 3 compound under the Chemical Weapons Convention 1993, due to being related to pinacolyl alcohol, which is used in the production of soman. It is also a controlled export in Australia Group member states.
John E. Franz is an organic chemist who discovered the herbicide glyphosate while working at Monsanto Company in 1970. The chemical became the active ingredient in Roundup, a broad-spectrum, post-emergence herbicide. Franz has earned acclaim and rewards for this breakthrough. He also has over 840 patents to his name worldwide.
Amine alkylation (amino-dehalogenation) is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution, and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but less so industrially, where alcohols are often preferred alkylating agents.

Saflufenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf weeds in crops including soybeans and corn.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.

Acifluorfen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to acifluorfen and its salts, via metabolic disposal by glutathione S-transferase. It is effective against broadleaf weeds and grasses and is used agriculturally on fields growing soybeans, peanuts, peas, and rice.

Aram Bagrati "Bagratovich" Nalbandyan was a Soviet and Armenian physicist, prominent in the field of physical chemistry, founder of the Institute of Chemical Physics in Yerevan, Armenia, and academician-secretary of the Chemical Department of the Armenian Academy of Sciences (AS). He is the author of more than 400 scientific articles and five monographs.
Pesticide degradation is the process by which a pesticide is transformed into a benign substance that is environmentally compatible with the site to which it was applied. Globally, an estimated 1 to 2.5 million tons of active pesticide ingredients are used each year, mainly in agriculture. Forty percent are herbicides, followed by insecticides and fungicides. Since their initial development in the 1940s, multiple chemical pesticides with different uses and modes of action have been employed. Pesticides are applied over large areas in agriculture and urban settings. Pesticide use, therefore, represents an important source of diffuse chemical environmental inputs.

Fluazifop is the common name used by the ISO for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.

Butafenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf and some grass weeds in crops including cereals and canola.

Tribenuron in the form of tribenuron-methyl is a sulfonylurea herbicide. Its mode of action is the inhibition of acetolactate synthase, group 2 of the Herbicide Resistance Action Committee's classification scheme.














