Related Research Articles

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.
Glycophorin C plays a functionally important role in maintaining erythrocyte shape and regulating membrane material properties, possibly through its interaction with protein 4.1. Moreover, it has previously been shown that membranes deficient in protein 4.1 exhibit decreased content of glycophorin C. It is also an integral membrane protein of the erythrocyte and acts as the receptor for the Plasmodium falciparum protein PfEBP-2.

Duffy antigen/chemokine receptor (DARC), also known as Fy glycoprotein (FY) or CD234, is a protein that in humans is encoded by the ACKR1 gene.

A gametocyte is a eukaryotic germ cell that divides by mitosis into other gametocytes or by meiosis into gametids during gametogenesis. Male gametocytes are called spermatocytes, and female gametocytes are called oocytes.

Primaquine is a medication used to treat and prevent malaria and to treat Pneumocystis pneumonia. Specifically it is used for malaria due to Plasmodium vivax and Plasmodium ovale along with other medications and for prevention if other options cannot be used. It is an alternative treatment for Pneumocystis pneumonia together with clindamycin. It is taken by mouth.

Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly. P. vivax is carried by the female Anopheles mosquito; the males do not bite.

Merozoitesurface proteins are both integral and peripheral membrane proteins found on the surface of a merozoite, an early life cycle stage of a protozoan. Merozoite surface proteins, or MSPs, are important in understanding malaria, a disease caused by protozoans of the genus Plasmodium. During the asexual blood stage of its life cycle, the malaria parasite enters red blood cells to replicate itself, causing the classic symptoms of malaria. These surface protein complexes are involved in many interactions of the parasite with red blood cells and are therefore an important topic of study for scientists aiming to combat malaria.
Subtelomeres are segments of DNA between telomeric caps and chromatin.
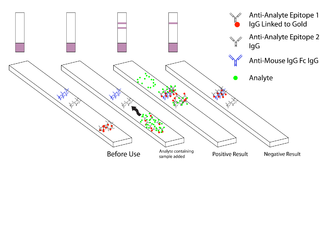
Malaria antigen detection tests are a group of commercially available rapid diagnostic tests of the rapid antigen test type that allow quick diagnosis of malaria by people who are not otherwise skilled in traditional laboratory techniques for diagnosing malaria or in situations where such equipment is not available. There are currently over 20 such tests commercially available. The first malaria antigen suitable as target for such a test was a soluble glycolytic enzyme Glutamate dehydrogenase. None of the rapid tests are currently as sensitive as a thick blood film, nor as cheap. A major drawback in the use of all current dipstick methods is that the result is essentially qualitative. In many endemic areas of tropical Africa, however, the quantitative assessment of parasitaemia is important, as a large percentage of the population will test positive in any qualitative assay.
Malaria vaccines are vaccines that prevent malaria, a mosquito-borne infectious disease which affected an estimated 249 million people globally in 85 malaria endemic countries and areas and caused 608,000 deaths in 2022. The first approved vaccine for malaria is RTS,S, known by the brand name Mosquirix. As of April 2023, the vaccine has been given to 1.5 million children living in areas with moderate-to-high malaria transmission. It requires at least three doses in infants by age 2, and a fourth dose extends the protection for another 1–2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.
Human genetic resistance to malaria refers to inherited changes in the DNA of humans which increase resistance to malaria and result in increased survival of individuals with those genetic changes. The existence of these genotypes is likely due to evolutionary pressure exerted by parasites of the genus Plasmodium which cause malaria. Since malaria infects red blood cells, these genetic changes are most common alterations to molecules essential for red blood cell function, such as hemoglobin or other cellular proteins or enzymes of red blood cells. These alterations generally protect red blood cells from invasion by Plasmodium parasites or replication of parasites within the red blood cell.
Pregnancy-associated malaria (PAM) or placental malaria is a presentation of malaria in pregnancy which is life-threatening to both pregnant women and unborn fetuses. PAM occurs when a pregnant woman contracts malaria, generally as a result of Plasmodium falciparum infection, and because she is pregnant, is at greater risk of associated complications such as placental malaria. Placental malaria interferes with the transmission of vital substances through the fetal placenta, which can result in stillbirths, miscarriages, and dangerously low birth weights.

In molecular biology, Duffy binding proteins are found in Plasmodium. Plasmodium vivax and Plasmodium knowlesi merozoites invade Homo sapiens erythrocytes that express Duffy blood group surface determinants. The Duffy receptor family is localised in micronemes, an organelle found in all organisms of the phylum Apicomplexa.
Circumsporozoite protein (CSP) is a secreted protein of the sporozoite stage of the malaria parasite and is the antigenic target of RTS,S and other malaria vaccines. The amino-acid sequence of CSP consists of an immunodominant central repeat region flanked by conserved motifs at the N- and C- termini that are implicated in protein processing as the parasite travels from the mosquito to the mammalian vector. The amino acid sequence of CSP was determined in 1984.
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells that are infected by the malarial parasite Plasmodium falciparum. PfEMP1 is synthesized during the parasite's blood stage inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with P. falciparum. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes called var. Each P. falciparum is able to switch on and off specific var genes to produce a functionally different protein, thereby evading the host's immune system. RBCs carrying PfEMP1 on their surface stick to endothelial cells, which facilitates further binding with uninfected RBCs, ultimately helping the parasite to both spread to other RBCs as well as bringing about the fatal symptoms of P. falciparum malaria.
Yagya Dutta Sharma is an Indian molecular biologist, professor and head of the department of biotechnology at the All India Institute of Medical Sciences, Delhi. An elected fellow of all three major Indian science academies — Indian National Science Academy, Indian Academy of Sciences, and National Academy of Sciences, India — Sharma is known for his research on the molecular biology of malaria. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology for his contributions to medical sciences in 1994.
Alan Frederick Cowman AC, FRS, FAA, CorrFRSE, FAAHMS, FASP, FASM is an internationally acclaimed malaria researcher whose work specialises in researching the malaria-causing parasite, Plasmodium falciparum, and the molecular mechanisms it uses to evade host responses and antimalarial drugs. As of May 2024, he is the deputy directory and Laboratory Head of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne, and his laboratory continues to work on understanding how Plasmodium falciparum, infects humans and causes disease. He was elected as a fellow of the Royal Society in 2011 and awarded the Companion of the Order of Australia in 2019 for his "eminent service to the biological sciences, notably to molecular parasitology, to medical research and scientific education, and as a mentor."

The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, but the identification of this part associated with a DnaJ domain in P. falciparum RESA has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc.
Reticulocyte binding protein homologs (RHs) are a superfamily of proteins found in Plasmodium responsible for cell invasion. Together with the family of erythrocyte binding-like proteins (EBLs) they make up the two families of invasion proteins universal to Plasmodium. The two families function cooperatively.
References
- 1 2 "People: Wai-Hong Tham". WEHI. 15 July 2019. Retrieved 10 Dec 2019.
- 1 2 Holland, Daryl (2018-09-13). "Associate Professor Wai-Hong Tham wins 2018 David Syme Research Prize". Faculty of Science. Retrieved 2019-12-10.
- ↑ Dinesh-Kumar, S. P.; Tham, Wai-Hong; Baker, Barbara J. (2000-12-19). "Structure–function analysis of the tobacco mosaic virus resistance gene N". Proceedings of the National Academy of Sciences. 97 (26): 14789–14794. Bibcode:2000PNAS...9714789D. doi: 10.1073/pnas.97.26.14789 . ISSN 0027-8424. PMC 18997 . PMID 11121079.
- 1 2 epaine (2017-05-19). "Former graduate student Wai-Hong Tham named International Research Scholar". molbio.princeton.edu. Retrieved 2019-12-10.
- ↑ Tham, Wai-Hong; Wyithe, J. Stuart B.; Ferrigno, Paul Ko; Silver, Pamela A.; Zakian, Virginia A. (2001-07-01). "Localization of Yeast Telomeres to the Nuclear Periphery Is Separable from Transcriptional Repression and Telomere Stability Functions". Molecular Cell. 8 (1): 189–199. doi: 10.1016/S1097-2765(01)00287-8 . ISSN 1097-2765. PMID 11511372.
- ↑ Marston, Adele L.; Tham, Wai-Hong; Shah, Hiral; Amon, Angelika (2004-02-27). "A Genome-Wide Screen Identifies Genes Required for Centromeric Cohesion". Science. 303 (5662): 1367–1370. Bibcode:2004Sci...303.1367M. doi:10.1126/science.1094220. ISSN 0036-8075. PMID 14752166. S2CID 36734853.
- ↑ Hochwagen, Andreas; Tham, Wai-Hong; Brar, Gloria A.; Amon, Angelika (2005-09-23). "The FK506 Binding Protein Fpr3 Counteracts Protein Phosphatase 1 to Maintain Meiotic Recombination Checkpoint Activity". Cell. 122 (6): 861–873. doi: 10.1016/j.cell.2005.07.010 . ISSN 0092-8674. PMID 16179256. S2CID 6731104.
- ↑ Tham, Wai-Hong; Wilson, Danny W.; Lopaticki, Sash; Schmidt, Christoph Q.; Tetteh-Quarcoo, Patience B.; Barlow, Paul N.; Richard, Dave; Corbin, Jason E.; Beeson, James G.; Cowman, Alan F. (2010-10-05). "Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand". Proceedings of the National Academy of Sciences. 107 (40): 17327–17332. Bibcode:2010PNAS..10717327T. doi: 10.1073/pnas.1008151107 . ISSN 0027-8424. PMC 2951459 . PMID 20855594.
- ↑ Tham, Wai-Hong; Schmidt, Christoph Q.; Hauhart, Richard E.; Guariento, Mara; Tetteh-Quarcoo, Patience B.; Lopaticki, Sash; Atkinson, John P.; Barlow, Paul N.; Cowman, Alan F. (2011-08-18). "Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes". Blood. 118 (7): 1923–1933. doi:10.1182/blood-2011-03-341305. ISSN 0006-4971. PMC 3158720 . PMID 21685372.
- ↑ Tham, Wai-Hong; Lim, Nicholas T. Y.; Weiss, Greta E.; Lopaticki, Sash; Ansell, Brendan R. E.; Bird, Megan; Lucet, Isabelle; Dorin-Semblat, Dominique; Doerig, Christian; Gilson, Paul R.; Crabb, Brendan S. (2015-12-22). "Plasmodium falciparum Adhesins Play an Essential Role in Signalling and Activation of Invasion into Human Erythrocytes". PLOS Pathogens. 11 (12): e1005343. doi: 10.1371/journal.ppat.1005343 . ISSN 1553-7374. PMC 4687929 . PMID 26694741.
- ↑ Gruszczyk, Jakub; Kanjee, Usheer; Chan, Li-Jin; Menant, Sébastien; Malleret, Benoit; Lim, Nicholas T. Y.; Schmidt, Christoph Q.; Mok, Yee-Foong; Lin, Kai-Min; Pearson, Richard D.; Rangel, Gabriel (2018-01-05). "Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax". Science. 359 (6371): 48–55. Bibcode:2018Sci...359...48G. doi:10.1126/science.aan1078. ISSN 0036-8075. PMC 5788258 . PMID 29302006.
- ↑ "Aussies solve malaria mystery". www.theaustralian.com.au. 2018-01-04. Retrieved 2019-12-10.
- ↑ "Aussie breakthrough could lead to malaria vaccine - 9News". www.9news.com.au. Retrieved 2019-12-10.
- 1 2 Gruszczyk, Jakub; Huang, Rick K.; Chan, Li-Jin; Menant, Sébastien; Hong, Chuan; Murphy, James M.; Mok, Yee-Foong; Griffin, Michael D. W.; Pearson, Richard D.; Wong, Wilson; Cowman, Alan F. (July 2018). "Cryo-EM structure of an essential Plasmodium vivax invasion complex". Nature. 559 (7712): 135–139. Bibcode:2018Natur.559..135G. doi:10.1038/s41586-018-0249-1. ISSN 1476-4687. PMID 29950717. S2CID 49470612.
- 1 2 First malaria-human contact mapped with Nobel Prize-winning technology , retrieved 2019-12-10
- ↑ Melbourne, Andrew Trounson, University of (2018-06-28). "Exposing malaria's atomic machinery". Pursuit. Retrieved 2019-12-10.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ "Philanthropies Select 41 Scientists as International Research Scholars". HHMI.org. Retrieved 2019-12-10.
- ↑ "Health Victoria News - Department of Health Victoria Australia". www.health.vic.gov.au. Retrieved 2019-12-10.
- ↑ "Celebrating Discovery 2018". WEHI. Dec 2018. Retrieved 10 Dec 2019.
- ↑ "Malaria team named 2019 Eureka Prize winner". WEHI - Illuminate. Retrieved 10 Dec 2019.
- ↑ "Eureka Prize for Institute malaria team". Mirage News. 2019-08-28. Retrieved 2019-12-10.
- ↑ "2020 Awards Winners". Biochemistry Society. Retrieved 10 Dec 2019.
- ↑ http://www.vic.gov.au/professor-wai-hong-tham