Nociception is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal in order to trigger an appropriate defense response.

Itch is a sensation that causes the desire or reflex to scratch. Itch has resisted many attempts to be classified as any one type of sensory experience. Itch has many similarities to pain, and while both are unpleasant sensory experiences, their behavioral response patterns are different. Pain creates a withdrawal reflex, whereas itch leads to a scratch reflex.

The grey column refers to a somewhat ridge-shaped mass of grey matter in the spinal cord. This presents as three columns: the anterior grey column, the posterior grey column, and the lateral grey column, all of which are visible in cross-section of the spinal cord.

A nociceptor is a sensory neuron that responds to damaging or potentially damaging stimuli by sending "possible threat" signals to the spinal cord and the brain. The brain creates the sensation of pain to direct attention to the body part, so the threat can be mitigated; this process is called nociception.

Sensory neurons, also known as afferent neurons, are neurons in the nervous system, that convert a specific type of stimulus, via their receptors, into action potentials or graded potentials. This process is called sensory transduction. The cell bodies of the sensory neurons are located in the dorsal ganglia of the spinal cord.

Hyperalgesia is an abnormally increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves and can cause hypersensitivity to stimulus. Prostaglandins E and F are largely responsible for sensitizing the nociceptors. Temporary increased sensitivity to pain also occurs as part of sickness behavior, the evolved response to infection.
The withdrawal reflex is a spinal reflex intended to protect the body from damaging stimuli. The reflex rapidly coordinates the contractions of all the flexor muscles and the relaxations of the extensors in that limb causing sudden withdrawal from the potentially damaging stimulus. Spinal reflexes are often monosynaptic and are mediated by a simple reflex arc. A withdrawal reflex is mediated by a polysynaptic reflex resulting in the stimulation of many motor neurons in order to give a quick response.
Neuropathic pain is pain caused by damage or disease affecting the somatosensory system. Neuropathic pain may be associated with abnormal sensations called dysesthesia or pain from normally non-painful stimuli (allodynia). It may have continuous and/or episodic (paroxysmal) components. The latter resemble stabbings or electric shocks. Common qualities include burning or coldness, "pins and needles" sensations, numbness and itching.

Referred pain, also called reflective pain, is pain perceived at a location other than the site of the painful stimulus. An example is the case of angina pectoris brought on by a myocardial infarction, where pain is often felt in the left side of neck, left shoulder, and back rather than in the thorax (chest), the site of the injury. The International Association for the Study of Pain has not officially defined the term; hence several authors have defined it differently.
Neuralgia is pain in the distribution of one or more nerves, as in intercostal neuralgia, trigeminal neuralgia, and glossopharyngeal neuralgia.
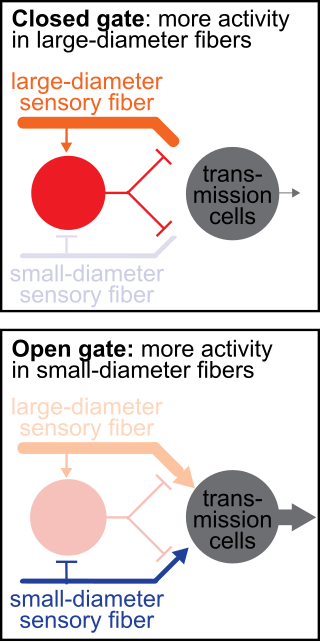
The gate control theory of pain asserts that non-painful input closes the nerve "gates" to painful input, which prevents pain sensation from traveling to the central nervous system.

Allodynia is a condition in which pain is caused by a stimulus that does not normally elicit pain. For example, bad sunburn can cause temporary allodynia, and touching sunburned skin, or running cold or warm water over it, can be very painful. It is different from hyperalgesia, an exaggerated response from a normally painful stimulus. The term is from Ancient Greek άλλοςállos "other" and οδύνηodúnē "pain".

The scratch reflex is a response to activation of sensory neurons whose peripheral terminals are located on the surface of the body. Some sensory neurons can be activated by stimulation with an external object such as a parasite on the body surface. Alternatively, some sensory neurons can respond to a chemical stimulus that produces an itch sensation. During a scratch reflex, a nearby limb reaches toward and rubs against the site on the body surface that has been stimulated. The scratch reflex has been extensively studied to understand the functioning of neural networks in vertebrates. Despite decades of research, key aspects of the scratch reflex are still unknown, such as the neural mechanisms by which the reflex is terminated.

Group C nerve fibers are one of three classes of nerve fiber in the central nervous system (CNS) and peripheral nervous system (PNS). The C group fibers are unmyelinated and have a small diameter and low conduction velocity, whereas Groups A and B are myelinated. Group C fibers include postganglionic fibers in the autonomic nervous system (ANS), and nerve fibers at the dorsal roots. These fibers carry sensory information.
Mechanosensation is the transduction of mechanical stimuli into neural signals. Mechanosensation provides the basis for the senses of light touch, hearing, proprioception, and pain. Mechanoreceptors found in the skin, called cutaneous mechanoreceptors, are responsible for the sense of touch. Tiny cells in the inner ear, called hair cells, are responsible for hearing and balance. States of neuropathic pain, such as hyperalgesia and allodynia, are also directly related to mechanosensation. A wide array of elements are involved in the process of mechanosensation, many of which are still not fully understood.
The rostral ventromedial medulla (RVM), or ventromedial nucleus of the spinal cord, is a group of neurons located close to the midline on the floor of the medulla oblongata (myelencephalon). The rostral ventromedial medulla sends descending inhibitory and excitatory fibers to the dorsal horn spinal cord neurons. There are 3 categories of neurons in the RVM: on-cells, off-cells, and neutral cells. They are characterized by their response to nociceptive input. Off-cells show a transitory decrease in firing rate right before a nociceptive reflex, and are theorized to be inhibitory. Activation of off-cells, either by morphine or by any other means, results in antinociception. On-cells show a burst of activity immediately preceding nociceptive input, and are theorized to be contributing to the excitatory drive. Neutral cells show no response to nociceptive input.
As long as humans have experienced pain, they have given explanations for its existence and sought soothing agents to dull or cease the painful sensation. Archaeologists have uncovered clay tablets dating back as far as 5,000 BC which reference the cultivation and use of the opium poppy to bring joy and cease pain. In 800 BC, the Greek writer Homer wrote in his epic, The Odyssey, of Telemachus, a man who used opium to soothe his pain and forget his worries. While some cultures researched analgesics and allowed or encouraged their use, others perceived pain to be a necessary, integral sensation. Physicians of the 19th century used pain as a diagnostic tool, theorizing that a greater amount of personally perceived pain was correlated to a greater internal vitality, and as a treatment in and of itself, inflicting pain on their patients to rid the patient of evil and unbalanced humors. This article focuses both on the history of how pain has been perceived across time and culture, but also how malleable an individual's perception of pain can be due to factors like situation, their visual perception of the pain, and previous history with pain.
Tactile induced analgesia is the phenomenon where concurrent touch and pain on the skin reduces the intensity of pain that is felt.
Sandra M. Garraway is a Canadian-American neuroscientist and assistant professor of physiology in the Department of Physiology at Emory University School of Medicine in Atlanta, Georgia. Garraway is the director of the Emory Multiplex Immunoassay Core (EMIC) where she assists researchers from both academia and industry to perform, analyze, and interpret their multiplexed immunoassays. Garraway studies the neural mechanisms of spinal nociceptive pain after spinal cord injury and as a postdoctoral researcher she discovered roles for both BDNF and ERK2 in pain sensitization and developed novel siRNA technology to inhibit ERK2 as a treatment for pain.

Nociplastic pain or central sensitisation is a type of pain which is mechanically different from the normal nociceptive pain caused by inflammation and tissue damage or the neuropathic pain which results from nerve damage. It may occur in combination with the other types of pain or in isolation. Its location may be generalised or multifocal and it can be more intense than would be expected from any associated physical cause.












