
Penicillins are a group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for various bacterial infections, though many types of bacteria have developed resistance following extensive use.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

The Peterson olefination is the chemical reaction of α-silyl carbanions with ketones to form a β-hydroxysilane (2) which eliminates to form alkenes (3).
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene or the sulfur analog of THF. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is an acid-labile protecting group used in organic synthesis.
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.
2-Methyl-2-nitrosopropane (MNP or t-nitrosobutane) is the organic compound with the formula (CH3)3CNO. It is a blue liquid that is used in chemical research as a spin trap, i.e. it binds to radicals.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.
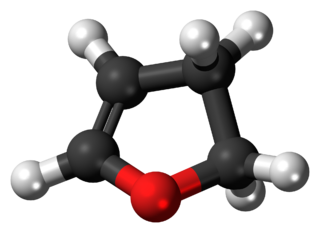
2,3-Dihydrofuran is a heterocyclic compound with the formula C4H6O. It is isomeric with 2,5-dihydrofuran. 2,3-Dihydrofuran is one of the simplest enol ethers. It is a colorless volatile liquid.
Melanie Sarah Sanford is an American chemist, currently the Moses Gomberg Distinguished University Professor of Chemistry and Arthur F. Thurnau Professor of Chemistry at the University of Michigan. She is a Fellow for the American Association for the Advancement of Science, and was elected a member of the National Academy of Sciences and the American Academy of Arts and Sciences in 2016. She has served as an executive editor of the Journal of the American Chemical Society since 2021, having been an associate editor of the since 2014.
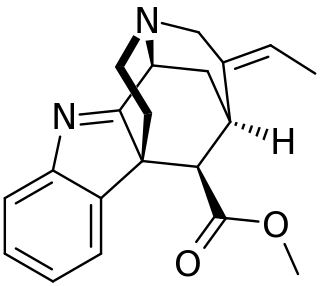
Strictamine is an alkaloid isolated from Alstonia scholaris.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
Shiina macrolactonization is an organic chemical reaction that synthesizes cyclic compounds by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic cyclization method using Lewis acid catalyst, and, in 2002, a basic cyclization using nucleophilic catalyst.
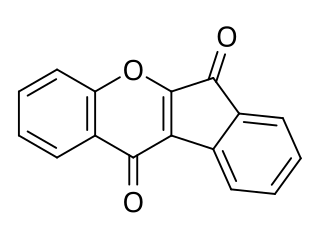
Wrightiadione is an isoflavone that occurs in the plant Wrightia tomentosa and can also be synthesized. It is a novel template for the TrkA kinase inhibitors.
Streptomyces nigrescens is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces nigrescens produces 5-alkyl-1,2,3,4-tetrahydroquinolines and the antibiotics phoslactomycin A - F.
Shiina esterification is an organic chemical reaction that synthesizes carboxylic esters from nearly equal amounts of carboxylic acids and alcohols by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic coupling method using Lewis acid, and, in 2002, a basic esterification using nucleophilic catalyst.
Vibralactones are a group of related terpenoid chemical compounds, the first of which were isolated from the Basidiomycete Boreostereum vibrans in 2006. Additional vibralactones have also been isolated from the fungus Stereum hirsutum.

Rick L. Danheiser is an American organic chemist and is the Arthur C. Cope Professor of Chemistry at the Massachusetts Institute of Technology and chair of the MIT faculty. His research involves the invention of new methods for the synthesis of complex organic compounds. Danheiser is known for the Danheiser annulation and Danheiser benzannulation reactions.

Prescopranone is a key intermediate in the biosynthesis of scopranones. Prescopranone is the precursor to scopranone A, scopranone B, and scopranone C, which are produced by Streptomyces sp. BYK-11038.

Lysergine, also known as 9,10-didehydro-6,8β-dimethylergoline, is an ergot alkaloid and serotonin receptor agonist of the ergoline family. It is a minor constituent of ergot.










