
Oryzomys nelsoni is an extinct rodent of María Madre Island, Nayarit, Mexico. Within the genus Oryzomys of the family Cricetidae, it may have been most closely related to the mainland species O. albiventer. Since its first description in 1898, most authors have regarded it as a distinct species, but it has also been classified as a mere subspecies of the marsh rice rat (O. palustris).

Oryzomys is a genus of semiaquatic rodents in the tribe Oryzomyini living in southern North America and far northern South America. It includes eight species, two of which—the marsh rice rat (O. palustris) of the United States and O. couesi of Mexico and Central America—are widespread; the six others have more restricted distributions. The species have had eventful taxonomic histories, and most species were at one time included in the marsh rice rat; additional species may be recognized in the future. The name Oryzomys was established in 1857 by Spencer Fullerton Baird for the marsh rice rat and was soon applied to over a hundred species of American rodents. Subsequently, the genus gradually became more narrowly defined until its current contents were established in 2006, when ten new genera were established for species previously placed in Oryzomys.

Lundomys molitor, also known as Lund's amphibious rat or the greater marsh rat, is a semiaquatic rat species from southeastern South America.

Pseudoryzomys simplex, also known as the Brazilian false rice rat or false oryzomys, is a species of rodent in the family Cricetidae from south-central South America. It is found in lowland palm savanna and thorn scrub habitats. It is a medium-sized species, weighing about 50 grams (1.8 oz), with gray–brown fur, long and narrow hindfeet, and a tail that is about as long as the head and body. The IUCN has assessed its conservation status as being of least concern, although almost nothing is known about its diet or reproduction.

Transandinomys bolivaris, also known as the long-whiskered rice rat, is a rodent in the family Cricetidae. It is found in humid forest from northeastern Honduras to western Ecuador, up to 1,800 m (5,900 ft) above sea level. Since it was first described in 1901 from Ecuador, six scientific names have been introduced for it, but their common identity was not documented until 1998 and the species has long been known under the name Oryzomys bombycinus, described from Panama in 1912. The name Oryzomys bolivaris was used before it was moved to the new genus Transandinomys with Transandinomys talamancae in 2006.

Oryzomys gorgasi, also known as Gorgas's oryzomys or Gorgas's rice rat, is a rodent in the genus Oryzomys of family Cricetidae. First recorded in 1967, it is known from only a few localities, including a freshwater swamp in the lowlands of northwestern Colombia and a mangrove islet in northwestern Venezuela. It reportedly formerly occurred on the island of Curaçao off northwestern Venezuela; this extinct population has been described as a separate species, Oryzomys curasoae, but does not differ morphologically from mainland populations.

Mindomys hammondi, also known as Hammond's rice rat or Hammond's oryzomys, is an endangered species of rodent in the tribe Oryzomyini of family Cricetidae. Formerly considered to be related with Nectomys, Sigmodontomys, Megalomys, or Oryzomys, it is now placed in then genus Mindomys, but its relationships remain obscure; some evidence supports a placement near Oecomys or as a basal member of Oryzomyini.

Eremoryzomys polius, also known as the gray rice rat or the Marañon oryzomys, is a rodent species in the tribe Oryzomyini of the family Cricetidae. Discovered in 1912 and first described in 1913 by Wilfred Osgood, it was originally placed in Oryzomys and named Oryzomys polius. In 2006, a cladistic analysis found that it was not closely related to Oryzomys in the strict sense or to any other oryzomyine then known, so that it is now placed in its own genus, Eremoryzomys. The Brazilian genus Drymoreomys, named in 2011, is probably the closest relative of Eremoryzomys. Eremoryzomys has a limited distribution in the dry upper valley of the Marañón River in central Peru, but may yet contain more than one species.

Oryzomyini is a tribe of rodents in the subfamily Sigmodontinae of the family Cricetidae. It includes about 120 species in about thirty genera, distributed from the eastern United States to the southernmost parts of South America, including many offshore islands. It is part of the clade Oryzomyalia, which includes most of the South American Sigmodontinae.

Noronhomys vespuccii, also known as Vespucci's rodent, is an extinct rat species from the islands of Fernando de Noronha off northeastern Brazil. Italian explorer Amerigo Vespucci may have seen it on a visit to Fernando de Noronha in 1503, but it subsequently became extinct, perhaps because of the exotic rats and mice introduced by the first explorers of the island. Numerous but fragmentary fossil remains of the animal, of uncertain but probably Holocene age, were discovered in 1973 and described in 1999.

Thomasomys ucucha, also known as the ucucha thomasomys, is a rodent in the genus Thomasomys of the family Cricetidae. It is known only from high altitude forest and grassland habitats in the Cordillera Oriental of Ecuador. Seven other species of Thomasomys live in the same areas. First collected in 1903, T. ucucha was formally described as a new species in 2003 and most closely resembles T. hylophilus, which occurs further to the north. The species is listed as "vulnerable" in the IUCN Red List as a result of habitat destruction.

Carletonomys cailoi is an extinct rodent from the Pleistocene (Ensenadan) of Buenos Aires Province, Argentina. Although known only from a single maxilla with the first molar, its features are so distinctive that it is placed in its own genus, Carletonomys. Discovered in 1998 and formally described in 2008, it is part of a well-defined group of oryzomyine rodents that also includes Holochilus, Noronhomys, Lundomys, and Pseudoryzomys. This group is characterized by progressive semiaquatic specializations and a reduction in the complexity of molar morphology.

Juliomys anoblepas is a rodent in the genus Juliomys of the subfamily Sigmodontinae known from a single broken skull. The specimen was collected by Peter Wilhelm Lund in the caves of Lagoa Santa, Minas Gerais, Brazil, in the first half of the 19th century and described by Herluf Winge in 1888 as Calomys anoblepas. The species remained unstudied and its affinities unclear until 2011, when it was recognized as a member of the genus Juliomys, which includes three other species from southern Brazil and nearby Argentina and Paraguay. J. anoblepas is probably a separate extinct species of the genus, which is no longer found at Lagoa Santa.

Transandinomys is a genus of rodents in the tribe Oryzomyini of family Cricetidae. It includes two species—T. bolivaris and T. talamancae—found in forests from Honduras in Central America south and east to southwestern Ecuador and northwestern Venezuela in northern South America. Until 2006, its members were included in the genus Oryzomys, but phylogenetic analysis showed that they are not closely related to the type species of that genus, and they have therefore been placed in a new genus. They may be most closely related to genera like Hylaeamys and Euryoryzomys, which contain very similar species. Both species of Transandinomys have had eventful taxonomic histories.
Nephelomys moerex is a species of rodent in the genus Nephelomys of family Cricetidae. The type locality is at Mindo in western Ecuador, where it has been recorded together with three other rodents of the oryzomyine group, Sigmodontomys aphrastus, Mindomys hammondi, and Handleyomys alfaroi, as well as three opossums, Chironectes minimus and unidentified species of Didelphis and Marmosa. Mindo is a "tiny agricultural community" located at 0°02'S, 78°48'W and 1,264 metres (4,150 ft) above sea level. It was originally described by Oldfield Thomas as a subspecies of Oryzomys albigularis. It remained synonymized under this species until it was recognized as a separate species when the genus Nephelomys was established for Oryzomys albigularis and related species in 2006.

Oecomys sydandersoni is an arboreal species of rodent in the genus Oecomys. It lives in forest patches in a small area in eastern Bolivia. It is a medium-sized species, weighing about 45 g (1.6 oz), with mostly grayish and brownish fur and short and broad hindfeet with well-developed pads.
In anatomy, posterolateral palatal pits are gaps at the sides of the back of the bony palate, near the last molars. Posterolateral palatal pits are present, in various degrees of development, in several members of the rodent family Cricetidae. Many members of the family lack them or have only simple pits, but Arvicolinae and Oryzomyini have more highly developed posterolateral palatal pits. Posterolateral palatal pits are also present in some other rodents, including Glis, Jaculus, Hystrix, Abrocoma, Ctenomys, Chinchilla, and Lagidium.
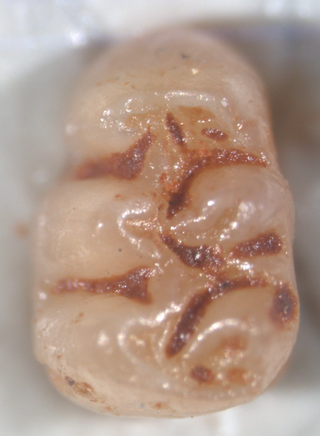
Agathaeromys is an extinct genus of oryzomyine rodents from the Pleistocene of Bonaire, Netherlands Antilles. Two species are known, which differ in size and some details of tooth morphology. The larger A. donovani, the type species, is known from hundreds of teeth that are probably 900,000 to 540,000 years old, found in four localities. A. praeuniversitatis, the smaller species, is known from 35 teeth found in a single fossil site, which is probably 540,000 to 230,000 years old.
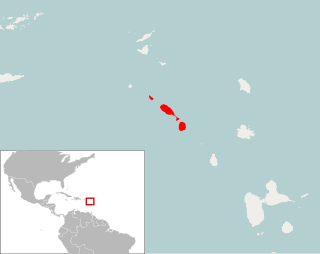
Pennatomys nivalis is an extinct oryzomyine rodent from the islands of Sint Eustatius, Saint Kitts, and Nevis in the Lesser Antilles. The only species in the genus Pennatomys, it is known from skeletal remains found in Amerindian archeological sites on all three islands, with dates ranging from 790–520 BCE to 900–1200 CE. No live specimens are known, but there are several historical records of rodents from Saint Kitts and Nevis that could conceivably refer to Pennatomys. The animal apparently belongs to a group within the tribe Oryzomyini that includes many other island-dwelling species.

Drymoreomys is a rodent genus in the tribe Oryzomyini that lives in the Atlantic Forest of Brazil. The single species, D. albimaculatus, is known only from the states of São Paulo and Santa Catarina and was not named until 2011. It lives in the humid forest on the eastern slopes of the Serra do Mar and perhaps reproduces year-round. Although its range is relatively large and includes some protected areas, it is patchy and threatened, and the discoverers recommend that the animal be considered "Near Threatened" on the IUCN Red List. Within Oryzomyini, Drymoreomys appears to be most closely related to Eremoryzomys from the Andes of Peru, a biogeographically unusual relationship, in that the two populations are widely separated and each is adapted to an arid or a moist environment.


















