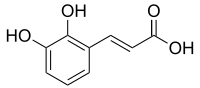
An acid is a molecule or ion capable of donating a proton (hydrogen ion H+) (a Brønsted–Lowry acid), or, alternatively, capable of forming a covalent bond with an electron pair (a Lewis acid).

Amino acids are organic compounds that contain amino (–NH2) and carboxyl (–COOH) functional groups, along with a side chain (R group) specific to each amino acid. The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N), although other elements are found in the side chains of certain amino acids. About 500 naturally occurring amino acids are known as of 1983 (though only 20 appear in the genetic code) and can be classified in many ways. They can be classified according to the core structural functional groups' locations as alpha- (α-), beta- (β-), gamma- (γ-) or delta- (δ-) amino acids; other categories relate to polarity, pH level, and side chain group type (aliphatic, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid residues form the second-largest component (water is the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis.
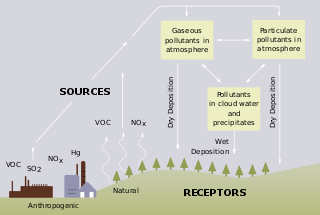
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions. It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Some governments have made efforts since the 1970s to reduce the release of sulfur dioxide and nitrogen oxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes, and sulphur dioxide is produced by volcanic eruptions. Acid rain has been shown to have adverse impacts on forests, freshwaters, and soils, killing insect and aquatic life-forms, causing paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and statues as well as having impacts on human health.

A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R–COOH or R-CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

The citric acid cycle (CAC) – also known as the TCA cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three segments of the citric acid cycle have been recognized.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually not found in organisms in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and they are important structural components for cells.

In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds; most commonly those that occur in living beings or in food.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.

Nucleic acids are biopolymers, or large biomolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is the ribose derivative deoxyribose, the polymer is DNA.
Nitric acid (HNO3), also known as aqua fortis (Latin for "strong water") and spirit of niter, is a highly corrosive mineral acid.
Omega−3 fatty acids, also called Omega-3 oils, ω−3 fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond, three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), found in plant oils, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both commonly found in marine oils. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. Common sources of plant oils containing ALA include walnut, edible seeds, clary sage seed oil, algal oil, flaxseed oil, Sacha Inchi oil, Echium oil, and hemp oil, while sources of animal omega−3 fatty acids EPA and DHA include fish, fish oils, eggs from chickens, squid oils and krill oil.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of cations and anions. Salts are composed of related numbers of cations and anions so that the product is electrically neutral. These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate ; and can be monatomic, such as fluoride (F−) or polyatomic, such as sulfate.

Sulfuric acid (American spelling) or sulphuric acid (Commonwealth spelling), also known as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with molecular formula H2SO4. It is a colorless, odorless and viscous liquid that is miscible with water at all concentrations.
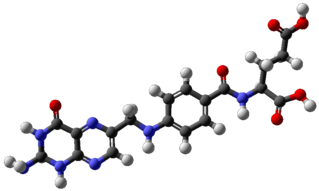
Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and storage. Folate is required for the body to make DNA and RNA and metabolise amino acids necessary for cell division. As humans cannot make folate, it is required in the diet, making it an essential nutrient. It occurs naturally in many foods. The recommended adult daily intake of folate in the U.S. is 400 micrograms from foods or dietary supplements.

Citric acid is a weak organic acid that has the molecular formula C6H8O7. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.

Formic acid, systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol.

Lactic acid is an organic acid. It has a molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate.

Acetic acid, systematically named ethanoic acid, is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water.

Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.