
Herbal teas, also known as herbal infusions and less commonly called tisanes, are beverages made from the infusion or decoction of herbs, spices, or other plant material in hot water. Oftentimes herb tea, or the plain term tea is used as a reference to all sorts of herbal teas. Some herbal blends contain actual tea.
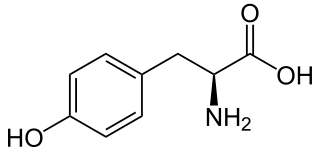
L-Tyrosine or tyrosine or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA.

Rooibos, meaning red bush, is a broom-like member of the plant family Fabaceae that grows in South Africa's fynbos biome.

Vitellaria paradoxa, commonly known as shea tree, shi tree, or vitellaria, is a tree of the family Sapotaceae. It is the only species in the genus Vitellaria, and is indigenous to Africa.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Alisma plantago-aquatica, also known as European water-plantain, common water-plantain or mad-dog weed, is a perennial flowering aquatic plant widespread across most of Europe and Asia, and apparently spread elsewhere in both the Old and New World.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

Ferulic acid is a hydroxycinnamic acid, an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans.

Rutin, also called rutoside, quercetin-3-O-rutinoside and sophorin, is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a citrus flavonoid found in a wide variety of plants including citrus.
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
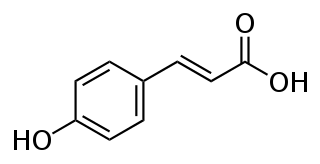
p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Cyclopia, the honeybush, or heuningbos in Afrikaans, is a genus of some 20 species of flowering plants in the legume family Fabaceae, subfamily Faboideae. Its description was published by the French botanist Étienne Pierre Ventenat in 1808. The name Ibbetsonia, published two years later, is regarded as a synonym of this genus; John Sims had commemorated the physiologist Agnes Ibbetson with this name.

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel of tea. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
9-cis-epoxycarotenoid dioxygenase (EC 1.13.11.51, nine-cis-epoxycarotenoid dioxygenase, NCED, AtNCED3, PvNCED1, VP14) is an enzyme in the biosynthesis of abscisic acid (ABA), with systematic name 9-cis-epoxycarotenoid 11,12-dioxygenase. This enzyme catalyses the following chemical reaction

Alisma orientale, commonly known as Asian water plantain, is a flowering plant species in the genus Alisma found in Asia.
Rooibos wine is a variety of wine in which wood from the rooibos and honeybush plants is used instead of the traditional oak wood during the maturation phase. It has its origin in the Western Cape, South Africa. The wine has a unique flavor component and due to the anti-oxidative effect of the plants can be produced without the use of sulfur dioxide.

















