Tumor necrosis factor is a cytokine and member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain. It is the first cytokine to be described as an adipokine as secreted by adipose tissue.
12-O-Tetradecanoylphorbol-13-acetate (TPA), also commonly known as tetradecanoylphorbol acetate, tetradecanoyl phorbol acetate, and phorbol 12-myristate 13-acetate (PMA) is a diester of phorbol. It is a potent tumor promoter often employed in biomedical research to activate the signal transduction enzyme protein kinase C (PKC). The effects of TPA on PKC result from its similarity to one of the natural activators of classic PKC isoforms, diacylglycerol. TPA is a small molecule drug.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.

Avenanthramides are a group of phenolic alkaloids found mainly in oats, but also present in white cabbage butterfly eggs, and in fungus-infected carnation. A number of studies demonstrate that these natural products have anti-inflammatory, antioxidant, anti-itch, anti-irritant, and antiatherogenic activities. Oat kernel extracts with standardized levels of avenanthramides are used for skin, hair, baby, and sun care products. The name avenanthramides was coined by Collins when he reported the presence of these compounds in oat kernels. It was later found that three avenanthramides were the open-ring amides of avenalumins I, II, and III, which were previously reported as oat phytoalexins by Mayama and co-workers.

Phorbol is a natural, plant-derived organic compound. It is a member of the tigliane family of diterpenes. Phorbol was first isolated in 1934 as the hydrolysis product of croton oil, which is derived from the seeds of the purging croton, Croton tiglium. The structure of phorbol was determined in 1967. Various esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C. They mimic diacylglycerols, glycerol derivatives in which two hydroxyl groups have reacted with fatty acids to form esters. The most common and potent phorbol ester is 12-O-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in contexts such as models of carcinogenesis.
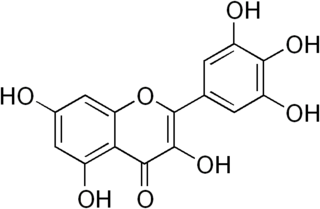
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.

Ursolic acid, is a pentacyclic triterpenoid identified in the epicuticular waxes of apples as early as 1920 and widely found in the peels of fruits, as well as in herbs and spices like rosemary and thyme.
Arachidonate 5-lipoxygenase inhibitors are compounds that slow or stop the action of the arachidonate 5-lipoxygenase enzyme, which is responsible for the production of inflammatory leukotrienes. The overproduction of leukotrienes is a major cause of inflammation in asthma, allergic rhinitis, and osteoarthritis.

Transcription factor p65 also known as nuclear factor NF-kappa-B p65 subunit is a protein that in humans is encoded by the RELA gene.

Inhibitor of nuclear factor kappa-B kinase subunit alpha (IKK-α) also known as IKK1 or conserved helix-loop-helix ubiquitous kinase (CHUK) is a protein kinase that in humans is encoded by the CHUK gene. IKK-α is part of the IκB kinase complex that plays an important role in regulating the NF-κB transcription factor. However, IKK-α has many additional cellular targets, and is thought to function independently of the NF-κB pathway to regulate epidermal differentiation.

Leukotriene B4 receptor 2, also known as BLT2, BLT2 receptor, and BLTR2, is an Integral membrane protein that is encoded by the LTB4R2 gene in humans and the Ltbr2 gene in mice.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.
A xanthine oxidase inhibitor is any substance that inhibits the activity of xanthine oxidase, an enzyme involved in purine metabolism. In humans, inhibition of xanthine oxidase reduces the production of uric acid, and several medications that inhibit xanthine oxidase are indicated for treatment of hyperuricemia and related medical conditions including gout. Xanthine oxidase inhibitors are being investigated for management of reperfusion injury.

Verbascoside is a polyphenol glycoside in which the phenylpropanoid caffeic acid and the phenylethanoid hydroxytyrosol form an ester and an ether bond respectively, to the rhamnose part of a disaccharide, namely β-(3′,4′-dihydroxyphenyl)ethyl-O-α-L-rhamnopyranosyl(1→3)-β-D-(4-O-caffeoyl)-glucopyranoside.
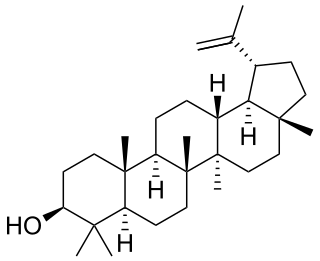
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity.

Gambogic acid is a xanthonoid that is derived from the brownish or orange resin from Garcinia hanburyi. Garcinia hanburyi is a small to medium-sized evergreen tree with smooth grey bark. It is native to Cambodia, southern Vietnam, and Thailand and has been successfully introduced in Singapore.
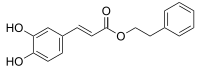
Ethyl caffeate is an ester of a hydroxycinnamic acid, a naturally occurring organic compound.

Celastrol (tripterine) is a chemical compound isolated from the root extracts of Tripterygium wilfordii and Tripterygium regelii. Celastrol is a pentacyclic nortriterpen quinone and belongs to the family of quinone methides. In mice, celastrol is an NR4A1 agonist that alleviates inflammation and induces autophagy. Also in mice, celastrol increase expression of IL1R1, which is the receptor for the cytokine interleukin-1 (IL-1). IL1R1 knock-out mice exposed to celastrol exhibit no leptin-sensitizing or anti-obesity effect.

Cereblon E3 ligase modulators, also known as immunomodulatory imide drugs (IMiDs), are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon (CRBN) is the protein targeted by this class of drugs.