Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.

Oxytocin is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. Present in animals since early stages of evolution, in humans it plays roles in behavior that include social bonding, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during labour. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in maternal bonding and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intensity and frequency and continues until the triggering activity ceases. A similar process takes place during lactation and during sexual activity.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.
Erepsin is a mixture of enzymes contained in a protein fraction found in the intestinal juices that digest peptones into amino acids. It is produced and secreted by the intestinal glands in the ileum and the pancreas, but it is also found widely in other cells. It is, however, a term now rarely used in scientific literature as more precise terms are preferred.
Cytosol alanyl aminopeptidase is an enzyme.

Leucyl/cystinyl aminopeptidase, also known as cystinyl aminopeptidase (CAP), insulin-regulated aminopeptidase (IRAP), human placental leucine aminopeptidase (PLAP), oxytocinase, and vasopressinase, is an enzyme of the aminopeptidase group that in humans is encoded by the LNPEP gene.
Protein metabolism denotes the various biochemical processes responsible for the synthesis of proteins and amino acids (anabolism), and the breakdown of proteins by catabolism.

Thermolysin is a thermostable neutral metalloproteinase enzyme produced by the Gram-positive bacteria Bacillus thermoproteolyticus. It requires one zinc ion for enzyme activity and four calcium ions for structural stability. Thermolysin specifically catalyzes the hydrolysis of peptide bonds containing hydrophobic amino acids. However thermolysin is also widely used for peptide bond formation through the reverse reaction of hydrolysis. Thermolysin is the most stable member of a family of metalloproteinases produced by various Bacillus species. These enzymes are also termed 'neutral' proteinases or thermolysin -like proteinases (TLPs).

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.

Puromycin-sensitive amino peptidase also known as cytosol alanyl aminopeptidase or alanine aminopeptidase (AAP) is an enzyme that in humans is encoded by the NPEPPS gene. It is used as a biomarker to detect damage to the kidneys, and that may be used to help diagnose certain kidney disorders. It is found at high levels in the urine when there are kidney problems.

RB-101 is a drug that acts as an enkephalinase inhibitor, which is used in scientific research.

Chromosome 9 open reading frame 3 (C9ORF3) also known as aminopeptidase O (APO) is an enzyme which in humans is encoded by the C9ORF3 gene. The protein encoded by this gene is an aminopeptidase which is most closely related in sequence to leukotriene A4 hydrolase (LTA4H). APO is a member of the M1 metalloproteinase family.

Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:

Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE). In mice, with the intracerebroventricular co-administration of a 50 µg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier) hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times. Kelatorphan also displays potent antinociceptive effects alone, and does not depress respiration, although at high doses it actually increases it.
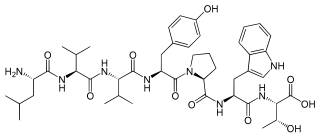
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

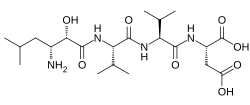
Tynorphin is a synthetic opioid peptide which is a potent and competitive inhibitor of the enkephalinase class of enzymes which break down the endogenous enkephalin peptides. It specifically inactivates dipeptidyl aminopeptidase III (DPP3) with very high efficacy, but also inhibits neutral endopeptidase (NEP), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE) to a lesser extent. It has a pentapeptide structure with the amino acid sequence Val-Val-Tyr-Pro-Trp (VVYPW).
Bacterial leucyl aminopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Oxytocinase is a type of enzyme that metabolizes the endogenous neuropeptide, oxytocin. The most well-characterized oxytocinase is leucyl/cystinyl aminopeptidase, which is also an enkephalinase. Other oxytocinases are also known. During pregnancy, oxytocinase plays a role in balancing concentration of oxytocin by degrading the oxytocin produced by the fetus, as production of oxytocin increases with growth of fetus. One study found that concentration level of oxytocinase increased progressively with gestational age until labor, which indicates that pregnancy development can be statistically evaluated by comparing oxytocinase levels.