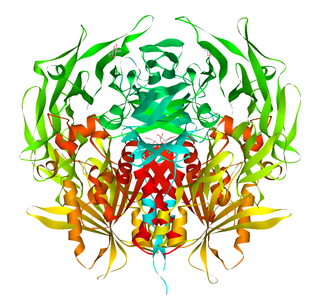
Dipeptidyl peptidase-4, also known as adenosine deaminase complexing protein 2 or CD26 is a protein that, in humans, is encoded by the DPP4 gene. DPP4 is related to FAP, DPP8, and DPP9. The enzyme was discovered in 1966 by Hopsu-Havu and Glenner, and as a result of various studies on chemism, was called dipeptidyl peptidase IV [DP IV].
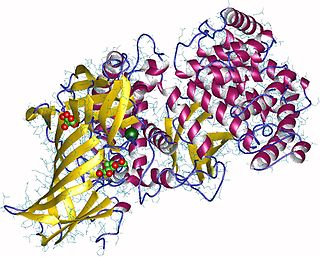
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the N-terminus (beginning), of proteins or peptides. They are found in many organisms; in the cell, they are found in many organelles, in the cytosol, and as membrane proteins. Aminopeptidases are used in essential cellular functions, and are often zinc metalloenzymes, containing a zinc cofactor.
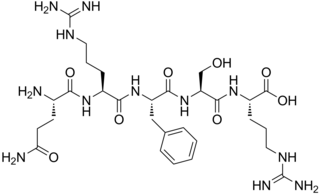
Opiorphin is an endogenous chemical compound first isolated from human saliva. Initial research with mice shows the compound has a painkilling effect greater than that of morphine. It works by stopping the normal breakup of enkephalins, natural pain-killing opioids in the spinal cord. It is a relatively simple molecule consisting of a five-amino acid polypeptide, Gln-Arg-Phe-Ser-Arg (QRFSR).

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.
Glutamyl aminopeptidase (EC 3.4.11.7, aminopeptidase A, aspartate aminopeptidase, angiotensinase A, glutamyl peptidase, Ca2+-activated glutamate aminopeptidase, membrane aminopeptidase II, antigen BP-1/6C3 of mouse B lymphocytes, L-aspartate aminopeptidase, angiotensinase A2) is an enzyme encoded by the ENPEP gene. Glutamyl aminopeptidase has also recently been designated CD249 (cluster of differentiation 249).

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.

Dipeptidyl peptidase 8 is an enzyme that in humans is encoded by the DPP8 gene.
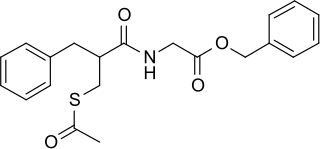
Racecadotril, also known as acetorphan, is an antidiarrheal medication which acts as a peripheral enkephalinase inhibitor. Unlike other opioid medications used to treat diarrhea, which reduce intestinal motility, racecadotril has an antisecretory effect — it reduces the secretion of water and electrolytes into the intestine. It is available in France and other European countries as well as most of South America and some South East Asian countries, but not in the United States. It is sold under the tradename Hidrasec, among others. Thiorphan is the active metabolite of racecadotril, which exerts the bulk of its inhibitory actions on enkephalinases.

RB-101 is a drug that acts as an enkephalinase inhibitor, which is used in scientific research.

Chromosome 9 open reading frame 3 (C9ORF3) also known as aminopeptidase O (APO) is an enzyme which in humans is encoded by the C9ORF3 gene. The protein encoded by this gene is an aminopeptidase which is most closely related in sequence to leukotriene A4 hydrolase (LTA4H). APO is a member of the M1 metalloproteinase family.
Dipeptidyl peptidase-4 inhibitors are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus. Inhibition of the DPP-4 enzyme prolongs and enhances the activity of incretins that play an important role in insulin secretion and blood glucose control regulation. Type 2 diabetes mellitus is a chronic metabolic disease that results from inability of the β-cells in the pancreas to secrete sufficient amounts of insulin to meet the body's needs. Insulin resistance and increased hepatic glucose production can also play a role by increasing the body's demand for insulin. Current treatments, other than insulin supplementation, are sometimes not sufficient to achieve control and may cause undesirable side effects, such as weight gain and hypoglycemia. In recent years, new drugs have been developed, based on continuing research into the mechanism of insulin production and regulation of the metabolism of sugar in the body. The enzyme DPP-4 has been found to play a significant role.
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:

Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE). In mice, with the intracerebroventricular co-administration of a 50 μg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier) hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times. Kelatorphan also displays potent antinociceptive effects alone, and does not depress respiration, although at high doses it actually increases it.
An enkephalinase inhibitor is a type of enzyme inhibitor which inhibits one or more members of the enkephalinase class of enzymes that break down the endogenous enkephalin opioid peptides. Examples include racecadotril, ubenimex (bestatin), RB-101, and D-phenylalanine, as well as the endogenous opioid peptides opiorphin and spinorphin. It also includes RB-3007, Semax and Selank. Analgesic, anticraving, antidepressant, anxiolytic, and antidiarrheal effects are common properties of enkephalinase inhibitors.
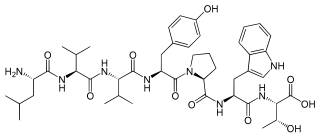
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.
Dipeptidyl-peptidase III is an enzyme. This enzyme catalyses the following chemical reaction
Oxytocinase is a type of enzyme that metabolizes the endogenous neuropeptide, oxytocin. The most well-characterized oxytocinase is leucyl/cystinyl aminopeptidase, which is also an enkephalinase. Other oxytocinases are also known. During pregnancy, oxytocinase plays a role in balancing concentration of oxytocin by degrading the oxytocin produced by the fetus, as production of oxytocin increases with growth of fetus. One study found that concentration level of oxytocinase increased progressively with gestational age until labor, which indicates that pregnancy development can be statistically evaluated by comparing oxytocinase levels.

Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3. It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase, bacterial leucyl aminopeptidase, leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and, to a lesser extent, glutamyl aminopeptidase, as well as other aminopeptidases. It does not inhibit arginyl aminopeptidase. Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin in vivo. It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides.
An endopeptidase inhibitor is a drug that inhibits one or more endopeptidase enzymes. Endopeptidases are one of two types of proteases, the other being exopeptidases. Endopeptidases cleave peptide bonds of non-terminal amino acids, whereas exopeptidases break terminal bonds, resulting in the release of a single amino acid or dipeptide from the peptide chain.

RB-120 is an orally active analog of the drug RB-101. It acts as an enkephalinase inhibitor, which is used in scientific research. Via intravenous administration, it is approximately three times as potent as RB-101 or twice as potent as the isolated (S,S) isomer of RB101. However, via i.p. administration it is approximately twice as potent as racemic RB-101 and about as potent as the isolated (S,S) isomer of RB101. During i.v. administration RB120 is approximately twice as weak as morphine in terms of analgesia; however, it is 16x weaker during i.p. and p.o. administration.













