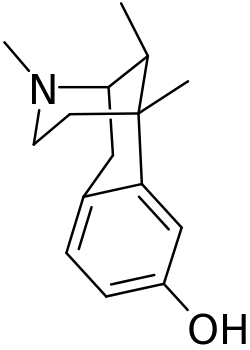
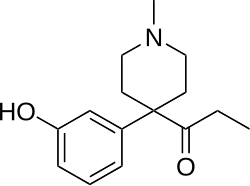
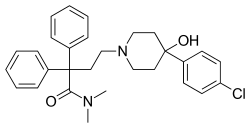
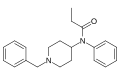
This is a list of opioids , opioid antagonists and inverse agonists.
Contents
- Opium and poppy straw derivatives
- Crude opiate extracts whole opium products
- Natural opiates
- Semisynthetics including Bentley compounds
- Active opiate metabolites
- Morphinans
- Morphinan series
- Others
- Benzomorphans
- 4-Phenylpiperidines
- Pethidines (meperidines)
- Prodines
- Ketobemidones
- Others 2
- Open chain opioids
- Amidones
- Methadols
- Moramides
- Thiambutenes
- Phenalkoxams
- Ampromides
- Others 3
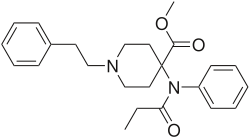
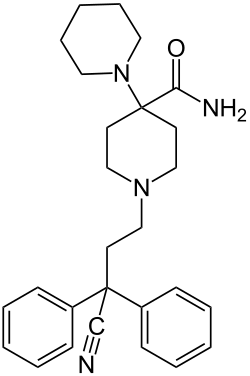
- Anilidopiperidines
- Oripavine derivatives
- Phenazepanes
- Pirinitramides
- Benzimidazoles
- Indoles
- Beta-Amino Ketones
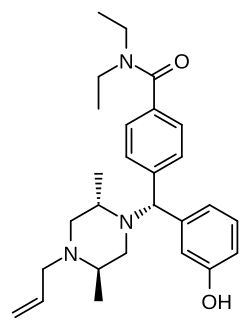
- Diphenylmethylpiperazines
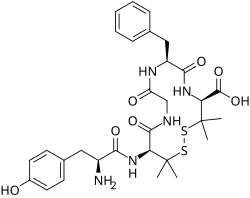
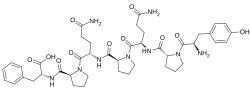
- Opioid peptides
- Dynorphins
- Endomorphins
- Endorphins
- Enkephalins
- Propeptides
- Others / unknown
- Others 4
- Opioid antagonists and inverse agonists
- Biased ligands
- Receptor heteromer targeting ligands
- Uncategorized opioids
- Combination drug formulations containing opioids
- See also
- References
- External links