Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is an analogue of fentanyl, of which it is structurally derivative. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans to image opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.

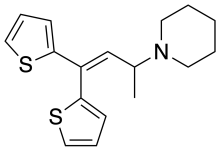
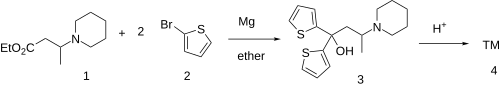
Ethylmethylthiambutene (N-ethyl-N-methyl-1-methyl-3,3-di-2-thienylallylamine; Emethibutin) is an opioid analgesic drug from the thiambutene family, around 1.3x the potency of morphine. It is under international control under Schedule I of the UN Single Convention On Narcotic Drugs 1961, presumably due to high abuse potential.

The Thiambutenes are a family of opioid analgesic drugs developed at the British research laboratory of Burroughs-Wellcome in the late 1940s. The parent compound thiambutene has no analgesic effects, but several compounds from this group are analgesics with around the same potency as morphine.

Pyrrolidinylthiambutene is an opioid analgesic drug from the thiambutene family with around 3/4 of the potency of morphine. It would be considered an illegal controlled substance analogue in some countries such as the US, Australia and New Zealand, but is legal in countries not possessing a controlled-substances-analog-act equivalent.

Butyrfentanyl or butyrylfentanyl is a potent short-acting synthetic opioid analgesic drug. It is an analog of fentanyl with around one quarter of its potency. One of the first mentions of this drug can be found in document written by The College on Problem of Drug Dependence, where it is mentioned as N-butyramide fentanyl analog. This document also states that the article describing its clinical effects was published in 1987. It is an agonist for the μ-opioid receptors.

U-47700, also known as U4, pink heroin, pinky, and pink, is an opioid analgesic drug developed by a team at Upjohn in the 1970s which has around 7.5 times the potency of morphine in animal models.
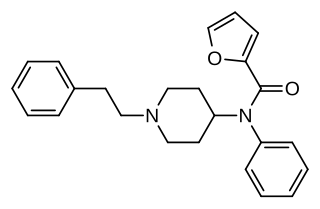
Furanylfentanyl (Fu-F) is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. It has an ED50 value of 0.02 mg/kg in mice. This makes it approximately one fifth as potent as fentanyl.
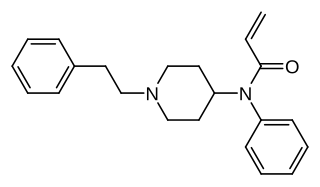
Acrylfentanyl (also known as acryloylfentanyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 (the half maximal inhibitory concentration for acrylfentanyl to displace naloxone) is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.
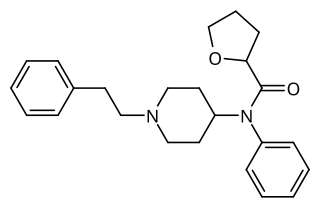
Tetrahydrofuranylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug, first appearing in Europe in late 2016.

Cyclopentylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug, mainly in Sweden and other Scandinavian countries.

4-Fluoroisobutyrylfentanyl (also known as 4-FIBF and p-FIBF) is an opioid analgesic that is an analog of butyrfentanyl and structural isomer of 4-Fluorobutyrfentanyl and has been sold online as a designer drug. It is closely related to 4-fluorofentanyl, which has an EC50 value of 4.2 nM for the human μ-opioid receptor. 4-fluoroisobutyrylfentanyl is a highly selective μ-opioid receptor agonist whose analgesic potency is almost ten times of that reported for morphine.

Valerylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. It has been seldom reported on illicit markets and there is little information about it, though it is believed to be less potent than butyrfentanyl but more potent than benzylfentanyl. In one study, it fully substituted for oxycodone and produced antinociception and oxycodone-like discriminative stimulus effects comparable in potency to morphine in mice, but failed to stimulate locomotor activity in mice at doses up to 100 mg/kg.

Cyclopropylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. Between June and December 2017, a total of 78 cyclopropylfentanyl-related deaths with analytical confirmation in post-mortem samples were reported by various European countries. Another 115 deaths involving cyclopropylfentanyl were reported from the United States in 2017.

Tetramethylcyclopropylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug.

Isofentanyl (3-methyl-benzylfentanyl) is an opioid analgesic that is an analog of fentanyl first invented in 1973, and which has been sold as a designer drug.

Metonitazene is an analgesic compound related to etonitazene, which was first reported in 1957, and has been shown to have approximately 1000 times the potency of morphine by central routes of administration, but if used orally it has been shown to have approximately 10 times the potency of morphine.

Butyrnorfentanyl or butyrylnorfentanyl is an inactive synthetic opioid analgesic drug precursor. It is an analog of fentanyl.
Utopioids are a class of synthetic opioid analgesic drugs first developed in the 1970s by the pharmaceutical company Upjohn. However, they were never marketed for medical use. Some compounds from this class have been used for scientific research as model kappa opioid receptor agonists. In the mid-2010s, one mu opioid receptor selective compound from this class, U-47700, re-emerged as a designer drug and became widely sold around the world for several years before being banned in various jurisdictions from 2016 onwards. Following the prohibition of U-47700, a number of related compounds have continued to appear on illicit drug markets. They are often marketed online or included as components in mixtures sold under the guise of "street heroin." U-47700 itself is the most potent mu opioid agonist from this class, around 7-10x the potency of morphine. Some other compounds such as 3,4-MDO-U-47700 and N-Ethyl-U-47700 retain similar mu selectivity but with lower potency similar to that of morphine, or have a mixture of mu and kappa mediated effects, such as U-48800. Most utopioid derivatives are however selective kappa agonists, which may have limited abuse potential as dissociative hallucinogens, but do not alleviate withdrawal distress in opioid dependent individuals or maintain addiction in a typical sense. Nevertheless, this has not stopped them from being sold as designer drugs, and a number of these compounds are now banned in many jurisdictions alongside U-47700 itself.