 | |
 | |
| Names | |
|---|---|
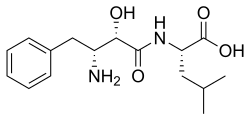
| IUPAC name N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanoyl]-L-leucine | |
| Systematic IUPAC name (2S)-2-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanamido]-4-methylpentanoic acid | |
| Other names Bestatin; N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.055.917 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C16H24N2O4 | |
| Molar mass | 308.378 g·mol−1 |
| Melting point | 245 °C (473 °F; 518 K) (decomposes) |
| Hazards | |
| GHS labelling: [2] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), [3] leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), [4] alanyl aminopeptidase (aminopeptidase M/N), [5] leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), [6] [7] and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia [8] and lymphedema. [9] It is derived from Streptomyces olivoreticuli . [10] Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.[ citation needed ]
Contents
