An analgesic drug, also called simply an analgesic, pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.

Morphine is a strong opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are available as MS-Contin, Kadian, and other brand names as well as generically.

Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work in the brain to produce a variety of effects, including pain relief. As a class of substances they act on opioid receptors to produce morphine-like effects.

Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.

Etonitazene, also known as EA-4941 or CS-4640, is a benzimidazole opioid, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.

Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.
Pantopon, also known as Opium Alkaloids Hydrochlorides, is a preparation of opiates made up of all of the alkaloids present in opium in their natural proportions as hydrochlorides salts. It can sometimes be tolerated by people who are allergic to morphine.
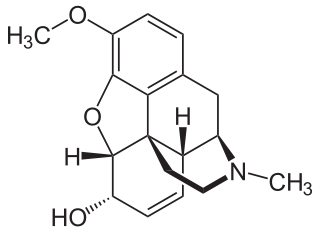
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children or adults. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.

Lofentanil or lofentanyl is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.
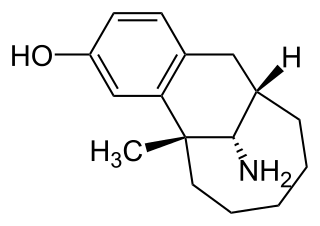
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
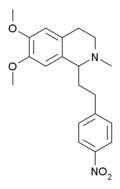
Ciramadol (WY-15,705) is an opioid analgesic that was developed in the late 1970s and is related to phencyclidine, tramadol, tapentadol and venlafaxine. It is a mixed agonist-antagonist for the μ-opioid receptor with relatively low abuse potential and a ceiling on respiratory depression which makes it a relatively safe drug. It has a slightly higher potency and effectiveness as an analgesic than codeine, but is weaker than morphine. Other side effects include sedation and nausea but these are generally less severe than with other similar drugs.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.
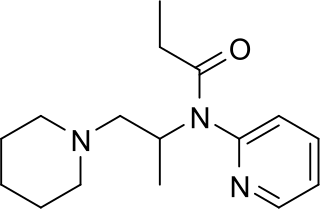
Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

7-PET is an opioid analgesic drug that has 300 times the potency of morphine by weight. It was discovered by K.W. Bentley and is related to the more well known oripavine derivative etorphine, which is used as a veterinary painkiller and anesthetic medication for the sedation of large animals such as elephants, giraffes, and rhinos. 7-PET itself has a 3-O-methyl ether which reduces potency, but the 3-OH derivative is around 2200 times more potent than morphine, almost the same potency as etorphine as a μ agonist, and unexpectedly the 3-hydrogen compound is also around the same potency of 2000 times morphine.

Oxymorphazone is an opioid analgesic drug related to oxymorphone. Oxymorphazone is a potent and long acting μ-opioid agonist which binds irreversibly to the receptor, forming a covalent bond which prevents it from detaching once bound. This gives it an unusual pharmacological profile, and while oxymorphazone is only around half the potency of oxymorphone, with higher doses the analgesic effect becomes extremely long lasting, with a duration of up to 48 hours. However, tolerance to analgesia develops rapidly with repeated doses, as chronically activated opioid receptors are rapidly internalised by β-arrestins, similar to the results of non-covalent binding by repeated doses of agonists with extremely high binding affinity such as lofentanil.
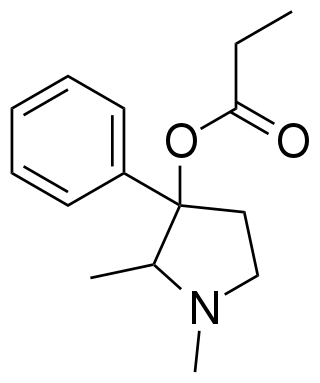
Prodilidine is an opioid analgesic which is a ring-contracted analogue of prodine. It has around the same analgesic efficacy as codeine, but is only around 1/3 the potency. It has little abuse potential.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others.

An opiate, is an alkaloid substance derived from opium It has a different meaning from the similar term opioid, used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America. Some, such as thebaine don't share the same effects and are not classified as controlled substances.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.