
A cloning vector is a small piece of DNA that can be stably maintained in an organism, and into which a foreign DNA fragment can be inserted for cloning purposes. The cloning vector may be DNA taken from a virus, the cell of a higher organism, or it may be the plasmid of a bacterium. The vector contains features that allow for the convenient insertion of a DNA fragment into the vector or its removal from the vector, for example through the presence of restriction sites. The vector and the foreign DNA may be treated with a restriction enzyme that cuts the DNA, and DNA fragments thus generated contain either blunt ends or overhangs known as sticky ends, and vector DNA and foreign DNA with compatible ends can then be joined by molecular ligation. After a DNA fragment has been cloned into a cloning vector, it may be further subcloned into another vector designed for more specific use.
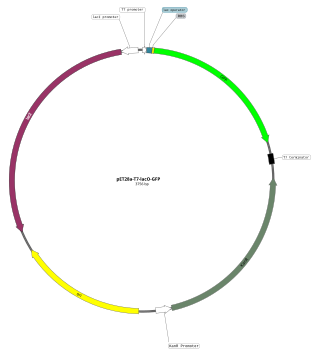
An expression vector, otherwise known as an expression construct, is usually a plasmid or virus designed for gene expression in cells. The vector is used to introduce a specific gene into a target cell, and can commandeer the cell's mechanism for protein synthesis to produce the protein encoded by the gene. Expression vectors are the basic tools in biotechnology for the production of proteins.
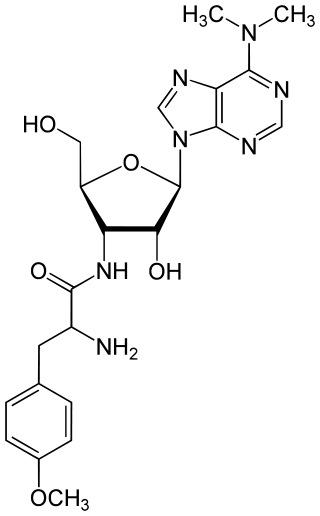
Puromycin is an antibiotic protein synthesis inhibitor which causes premature chain termination during translation.

Streptomyces is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have very large genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Different strains of the same species may colonize very diverse environments.
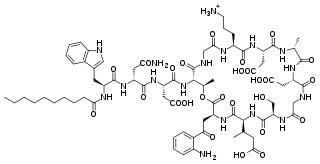
Daptomycin, sold under the brand name Cubicin among others, is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms.

Kanamycin A, often referred to simply as kanamycin, is an antibiotic used to treat severe bacterial infections and tuberculosis. It is not a first line treatment. It is used by mouth, injection into a vein, or injection into a muscle. Kanamycin is recommended for short-term use only, usually from 7 to 10 days. As with most antibiotics, it is ineffective in viral infections.

Glufosinate is a naturally occurring broad-spectrum herbicide produced by several species of Streptomyces soil bacteria. Glufosinate is a non-selective, contact herbicide, with some systemic action. Plants may also metabolize bialaphos and phosalacine, other naturally occurring herbicides, directly into glufosinate. The compound irreversibly inhibits glutamine synthetase, an enzyme necessary for the production of glutamine and for ammonia detoxification, giving it antibacterial, antifungal and herbicidal properties. Application of glufosinate to plants leads to reduced glutamine and elevated ammonia levels in tissues, halting photosynthesis and resulting in plant death.

In genetics, Flp-FRT recombination is a site-directed recombination technology, increasingly used to manipulate an organism's DNA under controlled conditions in vivo. It is analogous to Cre-lox recombination but involves the recombination of sequences between short flippase recognition target (FRT) sites by the recombinase flippase (Flp) derived from the 2 µ plasmid of baker's yeast Saccharomyces cerevisiae.

Hygromycin B is an antibiotic produced by the bacterium Streptomyces hygroscopicus. It is an aminoglycoside that kills bacteria, fungi and higher eukaryotic cells by inhibiting protein synthesis.
The pGreen plasmids are vectors for plant transformation. They were first described in 2000 as components of a novel T-DNA binary system. The supporting web page provides supplementary information and ongoing support to researchers to request their plasmid resources. As these plasmids have been taken up by the research community, the plasmids have been developed, expanding the resources available to the community.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
In enzymology, a carboxyvinyl-carboxyphosphonate phosphorylmutase (EC 2.7.8.23) is an enzyme that catalyzes the chemical reaction
In molecular cloning, a vector is any particle used as a vehicle to artificially carry a foreign nucleic sequence – usually DNA – into another cell, where it can be replicated and/or expressed. A vector containing foreign DNA is termed recombinant DNA. The four major types of vectors are plasmids, viral vectors, cosmids, and artificial chromosomes. Of these, the most commonly used vectors are plasmids. Common to all engineered vectors are an origin of replication, a multicloning site, and a selectable marker.
Resistance genes (R-Genes) are genes in plant genomes that convey plant disease resistance against pathogens by producing R proteins. The main class of R-genes consist of a nucleotide binding domain (NB) and a leucine rich repeat (LRR) domain(s) and are often referred to as (NB-LRR) R-genes or NLRs. Generally, the NB domain binds either ATP/ADP or GTP/GDP. The LRR domain is often involved in protein-protein interactions as well as ligand binding. NB-LRR R-genes can be further subdivided into toll interleukin 1 receptor (TIR-NB-LRR) and coiled-coil (CC-NB-LRR).

Molecular cloning is a set of experimental methods in molecular biology that are used to assemble recombinant DNA molecules and to direct their replication within host organisms. The use of the word cloning refers to the fact that the method involves the replication of one molecule to produce a population of cells with identical DNA molecules. Molecular cloning generally uses DNA sequences from two different organisms: the species that is the source of the DNA to be cloned, and the species that will serve as the living host for replication of the recombinant DNA. Molecular cloning methods are central to many contemporary areas of modern biology and medicine.
Streptomyces scabiei is a streptomycete bacterium species found in soils around the world. Unlike most of the 500 or so Streptomyces species it is a plant pathogen causing corky lesions to form on tuber and root crops as well as decreasing the growth of seedlings. Along with other closely related species it causes the potato disease common scab, which is an economically important disease in many potato growing areas. It was first described in 1892, being classified as a fungus, before being renamed in 1914 and again in 1948. Several other species of Streptomyces cause similar diseases to S. scabiei but other, more closely related species, do not.

Streptomyces hygroscopicus is a bacterial species in the genus Streptomyces. It was first described by Hans Laurits Jensen in 1931.
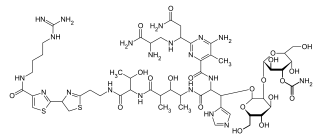
Zeocin is a trade name for a formulation of phleomycin D1, a glycopeptide antibiotic and one of the phleomycins from Streptomyces verticillus belonging to the bleomycin family of antibiotics. It is a broad-spectrum antibiotic that is effective against most aerobic organisms including bacteria, filamentous fungi, yeast, plant, and animal cells. It causes cell death by intercalating into DNA and inducing double stranded breaks of the DNA.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.

Dhara Mustard Hybrid-11, otherwise known as DMH - 11, is a genetically modified hybrid variety of the mustard species Brassica juncea. It was developed by Professor Deepak Pental from the University of Delhi, with the aim of reducing India's demand for edible oil imports. DMH - 11 was created through transgenic technology, primarily involving the Bar, Barnase and Barstar gene system. The Barnase gene confers male sterility, while the Barstar gene restores DMH - 11's ability to produce fertile seeds. The insertion of the third gene Bar, enables DMH - 11 to produce phosphinothricin-N- acetyl-transferase, the enzyme responsible for Glufosinate resistance. This hybrid mustard variety has come under intense public scrutiny, mainly due to concerns regarding DMH - 11's potential to adversely affect the environment as well as consumer health. DMH - 11 was found not to pose any food allergy risks, and has demonstrated increased yields over existing mustard varieties. Conflicting details and results regarding the field trials and safety evaluations conducted on DMH - 11 have delayed its approval for commercial cropping.














