Related Research Articles

Carbon is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.
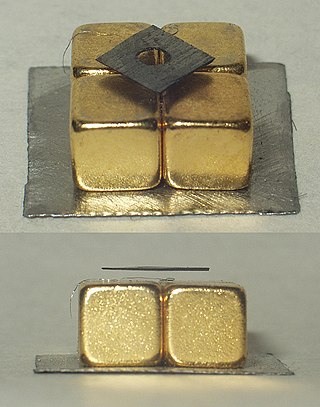
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, μ0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it entirely expels any magnetic field from its interior.

Ferromagnetism is a property of certain materials that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagnetic materials are noticeably attracted to a magnet, which is a consequence of their substantial magnetic permeability.

Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, magnetism is one of two aspects of electromagnetism.

Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.

Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).

Pulsed laser deposition (PLD) is a physical vapor deposition (PVD) technique where a high-power pulsed laser beam is focused inside a vacuum chamber to strike a target of the material that is to be deposited. This material is vaporized from the target which deposits it as a thin film on a substrate. This process can occur in ultra high vacuum or in the presence of a background gas, such as oxygen which is commonly used when depositing oxides to fully oxygenate the deposited films.
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amorphous carbon is often abbreviated to aC for general amorphous carbon, aC:H or HAC for hydrogenated amorphous carbon, or to ta-C for tetrahedral amorphous carbon.
In chemistry, a dangling bond is an unsatisfied valence on an immobilized atom. An atom with a dangling bond is also referred to as an immobilized free radical or an immobilized radical, a reference to its structural and chemical similarity to a free radical.
In magnetism, the Curie–Weiss law describes the magnetic susceptibility χ of a ferromagnet in the paramagnetic region above the Curie temperature:

Diamond-like carbon (DLC) is a class of amorphous carbon material that displays some of the typical properties of diamond. DLC is usually applied as coatings to other materials that could benefit from such properties.

Nanofoams are a class of nanostructured, porous materials (foams) containing a significant population of pores with diameters less than 100 nm. Aerogels are one example of nanofoam.

In nanotechnology, a carbon nanobud is a material that combines carbon nanotubes and spheroidal fullerenes, both allotropes of carbon, forming "buds" attached to the tubes. Carbon nanobuds were discovered and synthesized in 2006.
Molecule-based magnets (MBMs) or molecular magnets are a class of materials capable of displaying ferromagnetism and other more complex magnetic phenomena. This class expands the materials properties typically associated with magnets to include low density, transparency, electrical insulation, and low-temperature fabrication, as well as combine magnetic ordering with other properties such as photoresponsiveness. Essentially all of the common magnetic phenomena associated with conventional transition-metal magnets and rare-earth magnets can be found in molecule-based magnets. Prior to 2011, MBMs were seen to exhibit "magnetic ordering with Curie temperature (Tc) exceeding room temperature".
There are several known allotropes of oxygen. The most familiar is molecular oxygen, present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone. Others are:
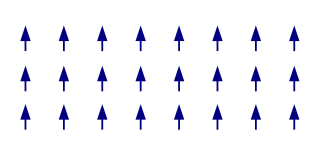
The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics.

In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom has a capacity to contain two electrons with opposite spins. As the formation of electron pairs is often energetically favourable, either in the form of a chemical bond or as a lone pair, unpaired electrons are relatively uncommon in chemistry, because an entity that carries an unpaired electron is usually rather reactive. In organic chemistry they typically only occur briefly during a reaction on an entity called a radical; however, they play an important role in explaining reaction pathways.
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin–orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin–orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.
References
- 1 2 Rode, A.V.; Hyde, S.T.; Gamaly, E.G.; Elliman, R.G.; McKenzie, D.R.; Bulcock, S. (1999). "Structural analysis of a carbon foam formed by high pulse-rate laser ablation". Applied Physics A: Materials Science & Processing. 69 (7): S755–S758. Bibcode:1999ApPhA..69S.755R. doi:10.1007/s003390051522. S2CID 96050247.
- 1 2 Rode, A.V.; Gamaly, E.G.; Luther-Davies, B. (2000-02-01). "Formation of cluster-assembled carbon nano-foam by high-repetition-rate laser ablation". Applied Physics A. 70 (2): 135–144. Bibcode:2000ApPhA..70..135R. doi:10.1007/s003390050025. hdl: 1885/35128 . ISSN 1432-0630. S2CID 98408906.
- ↑ Zani, A.; Dellasega, D.; Russo, V.; Passoni, M. (2013). "Ultra-low density carbon foams produced by pulsed laser deposition". Carbon. 56: 358–365. doi:10.1016/j.carbon.2013.01.029.
- 1 2 Blinc, R.; Arčon, D.; Umek, P.; Apih, T.; Milia, F.; Rode, A. V. (2007). "Carbon nanofoam as a potential hydrogen storage material". Physica Status Solidi B. 244 (11): 4308–4310. Bibcode:2007PSSBR.244.4308B. doi:10.1002/pssb.200776149. ISSN 1521-3951.
- ↑ Kenneth Chang (April 6, 2004). "A Flaky New Carbon: It's Feather Light and Magnetic". The New York Times .
- 1 2 3 Rode, A. V.; Gamaly, E. G.; Christy, A. G.; Fitz Gerald, J. G.; Hyde, S. T.; Elliman, R. G.; Luther-Davies, B.; Veinger, A. I.; Androulakis, J.; Giapintzakis, J. (2004-08-17). "Unconventional magnetism in all-carbon nanofoam". Physical Review B. 70 (5): 054407. arXiv: cond-mat/0310751 . Bibcode:2004PhRvB..70e4407R. doi:10.1103/PhysRevB.70.054407. S2CID 4011768.