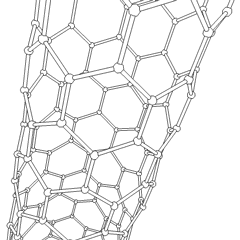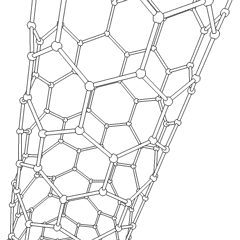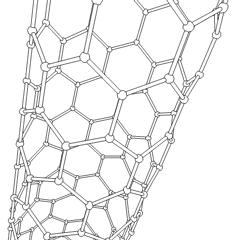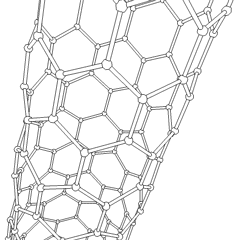
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometer range (nanoscale). They are one of the allotropes of carbon.

A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecules may be hollow spheres, ellipsoids, tubes, or other shapes.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to its three neighbors.

Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in the name reflects the notion of a small molecule trapped inside a shell. Two types of endohedral complexes exist: endohedral metallofullerenes and non-metal doped fullerenes.

The Prato reaction is a particular example of the well-known 1,3-dipolar cycloaddition of azomethine ylides to olefins. In fullerene chemistry this reaction refers to the functionalization of fullerenes and nanotubes. The amino acid sarcosine reacts with paraformaldehyde when heated at reflux in toluene to an ylide which reacts with a double bond in a 6,6 ring position in a fullerene via a 1,3-dipolar cycloaddition to yield a N-methylpyrrolidine derivative or pyrrolidinofullerene or pyrrolidino[[3,4:1,2]] [60]fullerene in 82% yield based on C60 conversion.
An organic superconductor is a synthetic organic compound that exhibits superconductivity at low temperatures.

Lanthanum carbide (LaC2) is a chemical compound. It is being studied in relation to the manufacture of certain types of superconductors and nanotubes.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

A geodesic polyarene in organic chemistry is a polycyclic aromatic hydrocarbon with curved convex or concave surfaces. Examples include fullerenes, nanotubes, corannulenes, helicenes and sumanene. The molecular orbitals of the carbon atoms in these systems are to some extent pyramidalized resulting a different pi electron density on either side of the molecule with consequences for reactivity.

In nanotechnology, a carbon nanobud is a material that combines carbon nanotubes and spheroidal fullerenes, both allotropes of carbon, forming "buds" attached to the tubes. Carbon nanobuds were discovered and synthesized in 2006.
Alex K. Zettl is an American experimental physicist, educator, and inventor.
Organic photovoltaic devices (OPVs) are fabricated from thin films of organic semiconductors, such as polymers and small-molecule compounds, and are typically on the order of 100 nm thick. Because polymer based OPVs can be made using a coating process such as spin coating or inkjet printing, they are an attractive option for inexpensively covering large areas as well as flexible plastic surfaces. A promising low cost alternative to conventional solar cells made of crystalline silicon, there is a large amount of research being dedicated throughout industry and academia towards developing OPVs and increasing their power conversion efficiency.

Rodney S. "Rod" Ruoff is an American physical chemist and nanoscience researcher. He is one of the world experts on carbon materials including carbon nanostructures such as fullerenes, nanotubes, graphene, diamond, and has had pioneering discoveries on such materials and others. Ruoff received his B.S. in chemistry from the University of Texas at Austin (1981) and his Ph.D. in chemical physics at the University of Illinois-Urbana (1988). After a Fulbright Fellowship at the MPI fuer Stroemungsforschung in Goettingen, Germany (1989) and postdoctoral work at the IBM T. J. Watson Research Center (1990–91), Ruoff became a staff scientist in the Molecular Physics Laboratory at SRI International (1991–1996). He is currently UNIST Distinguished Professor at the Ulsan National Institute of Science and Technology (UNIST), and the director of the Center for Multidimensional Carbon Materials, an Institute for Basic Science Center located at UNIST.

Titanium disulfide is an inorganic compound with the formula TiS2. A golden yellow solid with high electrical conductivity, it belongs to a group of compounds called transition metal dichalcogenides, which consist of the stoichiometry ME2. TiS2 has been employed as a cathode material in rechargeable batteries.

C70 fullerene is the fullerene molecule consisting of 70 carbon atoms. It is a cage-like fused-ring structure which resembles a rugby ball, made of 25 hexagons and 12 pentagons, with a carbon atom at the vertices of each polygon and a bond along each polygon edge. A related fullerene molecule, named buckminsterfullerene (C60 fullerene), consists of 60 carbon atoms.

Carbon peapod is a hybrid nanomaterial consisting of spheroidal fullerenes encapsulated within a carbon nanotube. It is named due to their resemblance to the seedpod of the pea plant. Since the properties of carbon peapods differ from those of nanotubes and fullerenes, the carbon peapod can be recognized as a new type of a self-assembled graphitic structure. Possible applications of nano-peapods include nanoscale lasers, single electron transistors, spin-qubit arrays for quantum computing, nanopipettes, and data storage devices thanks to the memory effects and superconductivity of nano-peapods.

Techniques have been developed to produce carbon nanotubes in sizable quantities, including arc discharge, laser ablation, high-pressure carbon monoxide disproportionation, and chemical vapor deposition (CVD). Most of these processes take place in a vacuum or with process gases. CVD growth of CNTs can occur in vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth are making CNTs more commercially viable.

Toxicology of carbon nanomaterials is the study of toxicity in carbon nanomaterials like fullerenes and carbon nanotubes.
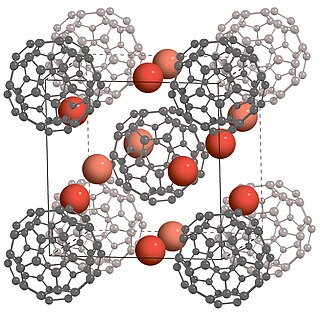
Fullerides are chemical compounds containing fullerene anions. Common fullerides are derivatives of the most common fullerenes, i.e. C60 and C70. The scope of the area is large because multiple charges are possible, i.e., [C60]n− (n = 1, 2...6), and all fullerenes can be converted to fullerides. The suffix "-ide" implies their negatively charged nature.

The solubility of fullerenes is generally low. Carbon disulfide dissolves 8g/L of C60, and the best solvent (1-chloronaphthalene) dissolves 53 g/L. up Still, fullerenes are the only known allotrope of carbon that can be dissolved in common solvents at room temperature. Besides those two, good solvents for fullerenes include 1,2-dichlorobenzene, toluene, p-xylene, and 1,2,3-tribromopropane. Fullerenes are highly insoluble in water, and practically insoluble in methanol.
















