
A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.

A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups. Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (η5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.

Nickelocene is the organonickel compound with the formula Ni(η5-C5H5)2. Also known as bis(cyclopentadienyl)nickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it does not yet have any known practical applications.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

Chromocene is the organochromium compound with the formula [Cr(C5H5)2]. Like structurally related metallocenes, chromocene readily sublimes in a vacuum and is soluble in non-polar organic solvents. It is more formally known as bis(η5-cyclopentadienyl)chromium(II).
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s. Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable.

Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests.
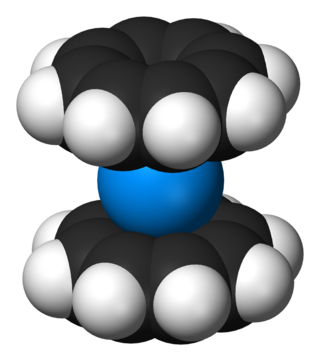
Organouranium chemistry is the science exploring the properties, structure, and reactivity of organouranium compounds, which are organometallic compounds containing a carbon to uranium chemical bond. The field is of some importance to the nuclear industry and of theoretical interest in organometallic chemistry.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.

Cyclopentadienyliron dicarbonyl iodide is an organoiron compound with the formula (C5H5)Fe(CO)2I. It is a dark brown solid that is soluble in common organic solvents. (C5H5)Fe(CO)2I, or FpI as it is often known, is an intermediate for the preparation of other organoiron compounds such as in ferraboranes.
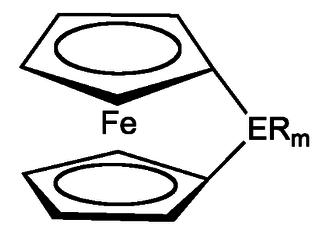
Ferrocenophanes, also called ansa ferrocenes, are organometallic compounds which are derived from ferrocene. They are a subset of ansa-metallocenes in which the metal is iron. In this compound class, the cyclopentadienyl ligands of the ferrocene are connected by one or more bridging groups. This hinders the rotation of the cyclopentadienyl ligands against one another and the reactivity of the complex with respect to the parent compound ferrocene is altered. A variety of organic and inorganic groups is suitable as bridging group.
Prof Peter Ludwig Israel Pauson FRSE FRIC (1925–2013) was a German–Jewish emigrant who settled in Britain and who is remembered for his contributions to chemistry, most notably the Pauson–Khand reaction and as joint discoverer of ferrocene.
Magnesocene, also known as bis(cyclopentadienyl)magnesium(II) and sometimes abbreviated as MgCp2, is an organometallic compound with the formula Mg(η5-C5H5)2. It is an example of an s-block main group sandwich compound, structurally related to the d-block element metallocenes, and consists of a central magnesium atom sandwiched between two cyclopentadienyl rings.
Thomas Joseph Kealy was an American chemist.
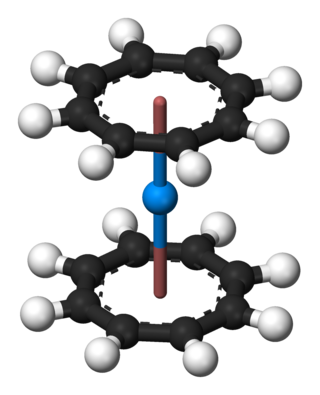
Organothorium chemistry describes the synthesis and properties of organothorium compounds, chemical compounds containing a carbon to thorium chemical bond.













