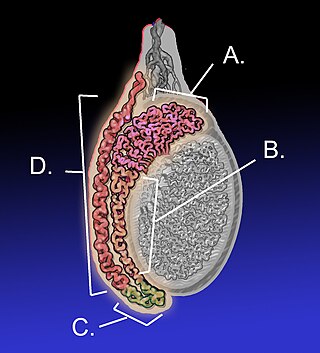
The epididymis is an elongated tubular structure attached to the posterior side of each one of the two male reproductive glands, the testicles. It is a single, narrow, tightly coiled tube in adult humans, 6 to 7 centimetres in length; uncoiled the tube would be approximately 6 m long. It connects the testicle to the vas deferens in the male reproductive system. The epididymis serves as an interconnection between the multiple efferent ducts at the rear of a testicle (proximally), and the vas deferens (distally). Its primary function is the storage, maturation and transport of sperm cells.
Capacitation is the penultimate step in the maturation of mammalian spermatozoa and is required to render them competent to fertilize an oocyte. This step is a biochemical event; the sperm move normally and look mature prior to capacitation. In vivo, capacitation occurs after ejaculation, when the spermatozoa leave the vagina and enter the upper female reproductive tract. The uterus aids in the steps of capacitation by secreting sterol-binding albumin, lipoproteins, and proteolytic and glycosidasic enzymes such as heparin.

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyse the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid “tail” and the glycerol molecule:

Spermiogenesis is the final stage of spermatogenesis, during which the spermatids develop into mature spermatozoa. At the beginning of the stage, the spermatid is a more or less circular cell containing a nucleus, Golgi apparatus, centriole and mitochondria; by the end of the process, it has radically transformed into an elongated spermatozoon, complete with a head, midpiece, and tail.
Ophanin is a toxin found in the venom of the King Cobra, which lives throughout South East Asia. This toxin belongs to the cysteine-rich secretory protein (CRISP) family. Ophanin weakly blocks the contraction of smooth muscles elicited by high potassium-induced depolarization, suggesting that it inhibits voltage-dependent calcium channels.
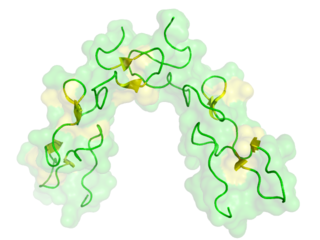
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.

Gloydius blomhoffii, commonly known as the mamushi, Japanese moccasin, Japanese pit viper, Qichun snake, Salmusa or Japanese mamushi, is a venomous pit viper species found in Japan. It was once considered to have 4 subspecies, but it is now considered monotypic.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.

Cysteine-rich secretory protein 3 is a cysteine-rich secretory protein that in humans is encoded by the CRISP3 gene.

Cysteine-rich secretory protein 1 is a cysteine-rich secretory protein that in humans is encoded by the CRISP1 gene.

Cysteine-rich secretory protein 2 is a cysteine-rich secretory protein that in humans is encoded by the CRISP2 gene.
Phoneutria keyserlingi is a species of spiders in the family Ctenidae, found in Brazil.

CatSper1, is a protein which in humans is encoded by the CATSPER1 gene. CatSper1 is a member of the cation channels of sperm family of protein. The four proteins in this family together form a Ca2+-permeant ion channel specific essential for the correct function of sperm cells.
Ablomin is a toxin present in the venom of the Japanese Mamushi snake, which blocks L-type voltage-gated calcium channels.
Triflin is a cysteine-rich secretory protein (CRISP), which is excreted by the venom gland of the Habu snake. Triflin reduces high potassium-induced smooth muscle contraction, suggesting a blocking effect on L-type calcium channels.
Piscivorin is a component of snake venom secreted by the Eastern Cottonmouth. It is a member of the cysteine-rich secretory protein (CRISP) family, which blocks voltage-dependent calcium channels.
Latisemin is a cysteine-rich secretory protein that can be isolated from the venom of the Black-banded sea krait, a sea snake indigenous to the warmer waters of the western Pacific Ocean. It is a toxin that inhibits cyclic nucleotide-gated ion channels and blocks L-type calcium channels, thereby reducing smooth muscle contraction.

Disintegrin and metalloproteinase domain-containing protein 7 is a protein that in humans is encoded by the ADAM7 gene. ADAM7 is an 85-kDa enzyme that is a member of the transmembrane ADAM protein family. Members of this family are membrane-anchored proteins structurally related to snake venom disintegrins, and have been implicated in a variety of biological processes involving cell-cell and cell-matrix interactions, including fertilization, muscle development, and neurogenesis. ADAM7 is important for the maturation of sperm cells in mammals. ADAM7 is also denoted as: ADAM_7, ADAM-7, EAPI, GP-83, and GP83.
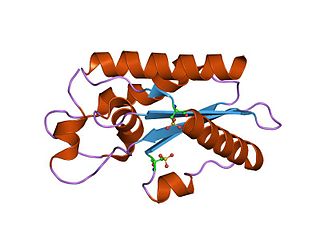
The CAP superfamily is a large superfamily of secreted proteins that are produced by a wide range of organisms, including prokaryotes and non-vertebrate eukaryotes.
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.











