
The Scoville scale is a measurement of pungency of chili peppers and other substances, recorded in Scoville heat units (SHU). It is based on the concentration of capsaicinoids, among which capsaicin is the predominant component.
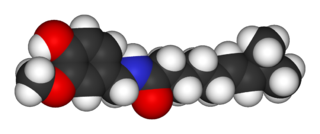
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is an active component of chili peppers, which are plants belonging to the genus Capsicum. It is a chemical irritant and neurotoxin for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related amides (capsaicinoids) are produced as secondary metabolites by chili peppers, probably as deterrents against certain mammals and fungi. Pure capsaicin is a hydrophobic, colorless, highly pungent, crystalline to waxy solid compound.

The bell pepper is the fruit of plants in the Grossum Group of the species Capsicum annuum. Cultivars of the plant produce fruits in different colors, including red, yellow, orange, green, white, chocolate, candy cane striped, and purple. Bell peppers are sometimes grouped with less pungent chili varieties as "sweet peppers". While they are botanically fruits—classified as berries—they are commonly used as a vegetable ingredient or side dish. Other varieties of the genus Capsicum are categorized as chili peppers when they are cultivated for their pungency, including some varieties of Capsicum annuum.

Chili peppers, from Nahuatl chīlli, are varieties of the berry-fruit of plants from the genus Capsicum, which are members of the nightshade family Solanaceae, cultivated for their pungency. Chili peppers are widely used in many cuisines as a spice to add "heat" to dishes. Capsaicin and related compounds known as capsaicinoids are the substances giving chili peppers their intensity when ingested or applied topically. Chili peppers exhibit a wide range of heat and flavor profiles. This diversity is the reason behind the availability of different types of paprika and chili powder, each offering its distinctive taste and heat level.

The jalapeño is a medium-sized chili pepper pod type cultivar of the species Capsicum annuum. A mature jalapeño chili is 5–10 cm (2–4 in) long and hangs down with a round, firm, smooth flesh of 25–38 mm wide. It can have a range of pungency, with Scoville heat units of 4,000 to 8,500. Commonly picked and consumed while still green, it is occasionally allowed to fully ripen and turn red, orange, or yellow. It is wider and generally milder than the similar Serrano pepper.

Capsicum pubescens is a plant of the genus Capsicum (pepper). The species name, pubescens, refers to the hairy leaves of this pepper. The hairiness of the leaves, along with the black seeds, make Capsicum pubescens distinguishable from other Capsicum species. Capsicum pubescens has pungent yellow, orange, red, green or brown fruits.

Capsicum annuum var. glabriusculum, a chili-pepper variety of Capsicum annuum, is native to southern North America and northern South America. Common names include chiltepín, Indian pepper, grove pepper, chiltepe, and chile tepín, as well as turkey, bird’s eye, or simply bird peppers. Tepín is derived from a Nahuatl word meaning "flea". This variety is the most likely progenitor of the domesticated C. annuum var. annuum. Another similar-sized pepper, 'Pequin' is often confused with tepin, although the tepin fruit is round to oval where as the pequin's fruit is oval with a point, and the leaves, stems and plant structures are very different on each plant.
Capsicum annuum is a species of the plant genus Capsicum native to southern North America, the Caribbean, and northern South America. This species is the most common and extensively cultivated of the five domesticated capsicums. The species encompasses a wide variety of shapes and sizes of peppers, including sweet bell peppers and some chili pepper varieties such as jalapeños, New Mexico chile, and cayenne peppers, all of which are nightshades. Cultivars descended from the wild American bird pepper are still found in warmer regions of the Americas. In the past, some woody forms of this species have been called C. frutescens, but the features that were used to distinguish those forms appear in many populations of C. annuum and are not consistently recognizable features in C. frutescens species.
Nordihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum).
Homodihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum). Like capsaicin it is an irritant. Homodihydrocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homodihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It produces "numbing burn" in the throat and is one of the most prolonged and difficult to rinse out. On the Scoville scale it has 8,600,000 SHU.

Homocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum). Like capsaicin it is an irritant. Homocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. On the Scoville scale it has 8,600,000 SHU. Homocapsaicin isolated from chili pepper has been found in two isomeric forms, both with a carbon-carbon double bond at the 6 position on the 10-carbon acyl chain. One isomer has an additional carbon, a methyl group, at the 8 position and the other has a methyl group at the 9 position. Homocapsaicin (6-ene-8-methyl) is the more abundant isomer. Homocapsaicin with the double bond at the 7 position has never been found in nature, though its structure is widely reported on the Internet and in the scientific literature. Details of this misidentification have been published.
Nonivamide, also called pelargonic acid vanillylamide or PAVA, is an organic compound and a capsaicinoid. It is an amide of pelargonic acid and vanillyl amine. It is present in chili peppers, but is commonly manufactured synthetically. It is more heat-stable than capsaicin.
Capsinoids are alkaloid substances naturally present in chili peppers. Although they are structurally similar to capsaicin, the substance that causes pungency in hot peppers, they largely lack that characteristic. Capsinoids have an estimated "hot taste threshold" which is about 1/1000 that of capsaicin. Capsinoids were not reported in the scientific literature until 1989, when biologists first isolated them in a unique variety of chili peppers, CH-19 Sweet, which does not contain capsaicin. Capsinoids include capsiate, dihydrocapsiate, and nordihydrocapsiate.

Shishito pepper is a sometimes hot East Asian pepper variety of the species Capsicum annuum.

Padrón pepper, also called Herbón pepper, is a landrace variety of pepper from the municipality of Padrón in northwestern Spain.

The Carolina Reaper chili pepper is a cultivar of the Capsicum chinense plant. Developed by American breeder Ed Currie, the pepper is red and gnarled, with a bumpy texture and small pointed tail. It was the hottest chili pepper in the world according to Guinness World Records from 2013 to 2023 before it was surpassed by Pepper X, which was also developed by Currie.

Vanillylamine is a chemical compound that is an intermediate in the biosynthesis of capsaicin. Vanillylamine is produced from vanillin by the enzyme vanillin aminotransferase. It is then converted with 8-methyl-6-nonenoic acid into capsaicin by the enzyme capsaicin synthase.

The ghost pepper, also known as bhut jolokia, is an interspecific hybrid chili pepper cultivated in Northeast India. It is a hybrid of Capsicum chinense and Capsicum frutescens.

Capsicum is a genus of flowering plants in the nightshade family Solanaceae, native to the Americas, cultivated worldwide for their chili pepper or bell pepper fruit.

Pepper X is a cultivar of Capsicum chili pepper bred by Ed Currie, creator of the Carolina Reaper. In 2023, Guinness World Records recognized it as the world's hottest pepper.



















