The small intestine or small bowel is an organ in the gastrointestinal tract where most of the absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion. The small intestine is about 5.5 metres long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is narrower in diameter.
Digestion is the breakdown of large insoluble food compounds into small water-soluble components so that they can be absorbed into the blood plasma. In certain organisms, these smaller substances are absorbed through the small intestine into the blood stream. Digestion is a form of catabolism that is often divided into two processes based on how food is broken down: mechanical and chemical digestion. The term mechanical digestion refers to the physical breakdown of large pieces of food into smaller pieces which can subsequently be accessed by digestive enzymes. Mechanical digestion takes place in the mouth through mastication and in the small intestine through segmentation contractions. In chemical digestion, enzymes break down food into the small compounds that the body can use.

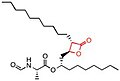
Orlistat, sold under the brand name Xenical among others, is a medication used to treat obesity. Its primary function is preventing the absorption of fats from the human diet by acting as a lipase inhibitor, thereby reducing caloric intake. It is intended for use in conjunction with a healthcare provider-supervised reduced-calorie diet.
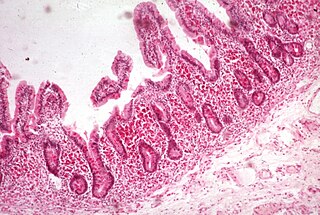
Malabsorption is a state arising from abnormality in absorption of food nutrients across the gastrointestinal (GI) tract. Impairment can be of single or multiple nutrients depending on the abnormality. This may lead to malnutrition and a variety of anaemias.
Steatorrhea is the presence of excess fat in feces. Stools may be bulky and difficult to flush, have a pale and oily appearance, and can be especially foul-smelling. An oily anal leakage or some level of fecal incontinence may occur. There is increased fat excretion, which can be measured by determining the fecal fat level. The definition of how much fecal fat constitutes steatorrhea has not been standardized.

Digestive enzymes take part in the chemical process of digestion, which follows the mechanical process of digestion. Food consists of macromolecules of proteins, carbohydrates, and fats that need to be broken down chemically by digestive enzymes in the mouth, stomach, pancreas, and duodenum, before being able to be absorbed into the bloodstream. Initial breakdown is achieved by chewing (mastication) and the use of digestive enzymes of saliva. Once in the stomach further mechanical churning takes place mixing the food with secreted gastric acid. Digestive gastric enzymes take part in some of the chemical process needed for absorption. Most of the enzymatic activity, and hence absorption takes place in the duodenum.
Fatty acid metabolism consists of various metabolic processes involving or closely related to fatty acids, a family of molecules classified within the lipid macronutrient category. These processes can mainly be divided into (1) catabolic processes that generate energy and (2) anabolic processes where they serve as building blocks for other compounds.

Lingual lipase is a member of a family of digestive enzymes called triacylglycerol lipases, EC 3.1.1.3, that use the catalytic triad of aspartate, histidine, and serine to hydrolyze medium and long-chain triglycerides into partial glycerides and free fatty acids. The enzyme, released into the mouth along with the saliva, catalyzes the first reaction in the digestion of dietary lipid, with diglycerides being the primary reaction product. However, due to the unique characteristics of lingual lipase, including a pH optimum 4.5–5.4 and its ability to catalyze reactions without bile salts, the lipolytic activity continues through to the stomach. Enzyme release is signaled by autonomic nervous system after ingestion, at which time the serous glands under the circumvallate and foliate lingual papillae on the surface of the tongue secrete lingual lipase to the grooves of the circumvallate and foliate papillae, co-localized with fat taste receptors. The hydrolysis of the dietary fats is essential for fat absorption by the small intestine, as long chain triacylglycerides cannot be absorbed, and as much as 30% of fat is hydrolyzed within 1 to 20 minutes of ingestion by lingual lipase alone.
Lipid metabolism is the synthesis and degradation of lipids in cells, involving the breakdown and storage of fats for energy and the synthesis of structural and functional lipids, such as those involved in the construction of cell membranes. In animals, these fats are obtained from food and are synthesized by the liver. Lipogenesis is the process of synthesizing these fats. The majority of lipids found in the human body from ingesting food are triglycerides and cholesterol. Other types of lipids found in the body are fatty acids and membrane lipids. Lipid metabolism is often considered the digestion and absorption process of dietary fat; however, there are two sources of fats that organisms can use to obtain energy: from consumed dietary fats and from stored fat. Vertebrates use both sources of fat to produce energy for organs such as the heart to function. Since lipids are hydrophobic molecules, they need to be solubilized before their metabolism can begin. Lipid metabolism often begins with hydrolysis, which occurs with the help of various enzymes in the digestive system. Lipid metabolism also occurs in plants, though the processes differ in some ways when compared to animals. The second step after the hydrolysis is the absorption of the fatty acids into the epithelial cells of the intestinal wall. In the epithelial cells, fatty acids are packaged and transported to the rest of the body.
Fatty acid synthase (FAS) is an enzyme that in humans is encoded by the FASN gene.

Lipstatin is a potent, irreversible inhibitor of pancreatic lipase. It is a natural product that was first isolated from the actinobacterium Streptomyces toxytricini.

In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis. It typically uses malonyl-CoA as a carbon source to elongate ACP-bound acyl species, resulting in the formation of ACP-bound β-ketoacyl species such as acetoacetyl-ACP.

Gastric lipase, also known as LIPF, is an enzymatic protein that, in humans, is encoded by the LIPF gene.

Fatty-acyl-CoA Synthase, or more commonly known as yeast fatty acid synthase, is an enzyme complex responsible for fatty acid biosynthesis, and is of Type I Fatty Acid Synthesis (FAS). Yeast fatty acid synthase plays a pivotal role in fatty acid synthesis. It is a 2.6 MDa barrel shaped complex and is composed of two, unique multi-functional subunits: alpha and beta. Together, the alpha and beta units are arranged in an α6β6 structure. The catalytic activities of this enzyme complex involves a coordination system of enzymatic reactions between the alpha and beta subunits. The enzyme complex therefore consists of six functional centers for fatty acid synthesis.

Triglyceride lipases are a family of lipolytic enzymes that hydrolyse ester linkages of triglycerides. Lipases are widely distributed in animals, plants and prokaryotes.

Antinutrients are natural or synthetic compounds that interfere with the absorption of nutrients. Nutrition studies focus on antinutrients commonly found in food sources and beverages. Antinutrients may take the form of drugs, chemicals that naturally occur in food sources, proteins, or overconsumption of nutrients themselves. Antinutrients may act by binding to vitamins and minerals, preventing their uptake, or inhibiting enzymes.
Cetilistat is a drug designed to treat obesity. It acts in the same way as the older drug orlistat (Xenical) by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are excreted undigested.

In biochemistry, lipase refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms.

Lipase inhibitors are substances used to reduce the activity of lipases found in the intestine. Lipases are secreted by the pancreas when fat is present. The primary role of lipase inhibitors is to decrease the gastrointestinal absorption of fats. Fats then tend to be excreted in feces rather than being absorbed to be used as a source of caloric energy, and this can result in weight loss in individuals. These inhibitors could be used for the treatment of obesity, which can subsequently lead to Type 2 diabetes and cardiovascular diseases if not managed. An example of a lipase inhibitor is orlistat.

The human digestive system consists of the gastrointestinal tract plus the accessory organs of digestion. Digestion involves the breakdown of food into smaller and smaller components, until they can be absorbed and assimilated into the body. The process of digestion has three stages: the cephalic phase, the gastric phase, and the intestinal phase.