
Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge, a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria. It is a potent functional analog of capsaicin, the active ingredient in chili peppers.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
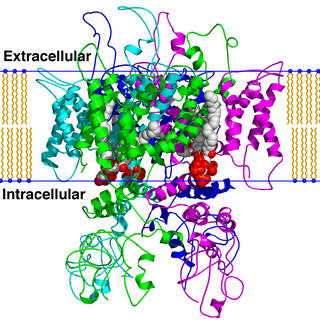
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Capsazepine is a synthetic antagonist of capsaicin. It is used as a biochemical tool in the study of TRPV ion channels.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

Transient receptor potential cation channel subfamily V member 4 is an ion channel protein that in humans is encoded by the TRPV4 gene.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

Transient receptor potential cation channel, subfamily V, member 3, also known as TRPV3, is a human gene encoding the protein of the same name.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. It is applied three times daily for a maximum of three months. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.

LASSBio-881 is a drug which acts as both a non-selective partial agonist of the CB1 and CB2 cannabinoid receptors, and also as an antagonist of the TRPV1 receptor, as well as having antioxidant effects. It has potent anti-inflammatory and anti-hyperalgesic effects in animal studies.

Vanillotoxins are neurotoxins found in the venom of the tarantula Psalmopoeus cambridgei. They act as agonists for the transient receptor potential cation channel subfamily V member 1 (TRPV1), activating the pain sensory system. VaTx1 and 2 also act as antagonists for the Kv2-type voltage-gated potassium channel (Kv2), inducing paralytic behavior in small animals.

The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.

Phenylacetylrinvanil (IDN-5890) is a synthetic analogue of capsaicin which acts as a potent and selective agonist for the TRPV1 receptor, with slightly lower potency than resiniferatoxin, though still around 300 times the potency of capsaicin. It is an amide of vanillylamine and ricinoleic acid, with the hydroxyl group on ricinoleic acid esterified with phenylacetic acid. It is used to study the function of the TRPV1 receptor and its downstream actions, and has also shown anti-cancer effects in vitro.

AMG-9810 is a drug which acts as a potent and selective antagonist for the TRPV1 receptor. It has analgesic and antiinflammatory effects and is used in scientific research, but has not been developed for medical use. It has high antagonist potency and good bioavailability and pharmacokinetics, and so has been used to study the role of TRPV1 in areas other than pain perception, such as its roles in the brain.

AMG-517 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It was developed as a potential treatment for chronic pain, but while it was an effective analgesic in animal studies it was dropped from human clinical trials at Phase I due to producing hyperthermia as a side effect, as well as poor water solubility. It is still used in scientific research into the function of the TRPV1 channel and its role in pain and inflammation, and has been used as a template for the design of several newer analogues which have improved properties.

SB-705498 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It has been evaluated in clinical trials for the treatment of rhinitis and chronic cough.

Double-knot toxin (DkTx), also known as Tau-theraphotoxin-Hs1a or Tau-TRTX-Hs1a, is a toxin found in the venom of the Chinese Bird spider, a tarantula species primarily living in the Guangxi province of China. This toxin, characterized by its bivalent structure of two Inhibitor Cysteine Knots (ICK), is thought to induce excruciating and long-lasting pain by activating the transient receptor potential vanilloid 1 (TRPV1) channel.


























