
A heart valve is a biological one-way valve that allows blood to flow in one direction through the chambers of the heart. Four valves are usually present in a mammalian heart and together they determine the pathway of blood flow through the heart. A heart valve opens or closes according to differential blood pressure on each side.

The aortic valve is a valve in the heart of humans and most other animals, located between the left ventricle and the aorta. It is one of the four valves of the heart and one of the two semilunar valves, the other being the pulmonary valve. The aortic valve normally has three cusps or leaflets, although in 1–2% of the population it is found to congenitally have two leaflets. The aortic valve is the last structure in the heart the blood travels through before stopping the flow through the systemic circulation.

A congenital heart defect (CHD), also known as a congenital heart anomaly, congenital cardiovascular malformation, and congenital heart disease, is a defect in the structure of the heart or great vessels that is present at birth. A congenital heart defect is classed as a cardiovascular disease. Signs and symptoms depend on the specific type of defect. Symptoms can vary from none to life-threatening. When present, symptoms are variable and may include rapid breathing, bluish skin (cyanosis), poor weight gain, and feeling tired. CHD does not cause chest pain. Most congenital heart defects are not associated with other diseases. A complication of CHD is heart failure.
A cyanotic heart defect is any congenital heart defect (CHD) that occurs due to deoxygenated blood bypassing the lungs and entering the systemic circulation, or a mixture of oxygenated and unoxygenated blood entering the systemic circulation. It is caused by structural defects of the heart such as right-to-left or bidirectional shunting, malposition of the great arteries, or any condition which increases pulmonary vascular resistance. The result may be the development of collateral circulation.
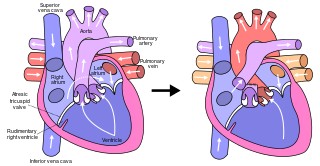
The Fontan procedure or Fontan–Kreutzer procedure is a palliative surgical procedure used in children with univentricular hearts. It involves diverting the venous blood from the inferior vena cava (IVC) and superior vena cava (SVC) to the pulmonary arteries. The procedure varies for differing congenital heart pathologies. For example in tricuspid atresia, the procedure can be done where the blood does not pass through the morphologic right ventricle; i.e., the systemic and pulmonary circulations are placed in series with the functional single ventricle. Whereas in hypoplastic left heart syndrome, the heart is more reliant on the more functional right ventricle to provide blood flow to the systemic circulation. The procedure was initially performed in 1968 by Francis Fontan and Eugene Baudet from Bordeaux, France, published in 1971, simultaneously described in 1971 by Guillermo Kreutzer from Buenos Aires, Argentina, and finally published in 1973.
The Rastelli procedure is an open heart surgical procedure developed by Italian physician and cardiac surgery researcher, Giancarlo Rastelli, in 1967 at the Mayo Clinic, and involves using a pulmonary or aortic homograft conduit to relieve pulmonary obstruction in double outlet right ventricle with pulmonary stenosis.
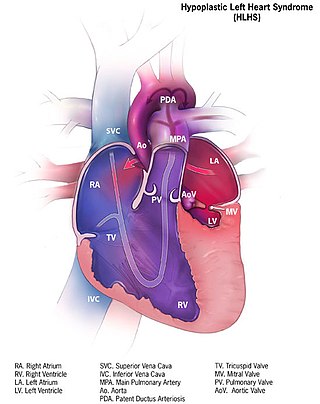
Hypoplastic left heart syndrome (HLHS) is a rare congenital heart defect in which the left side of the heart is severely underdeveloped and incapable of supporting the systemic circulation. It is estimated to account for 2-3% of all congenital heart disease. Early signs and symptoms include poor feeding, cyanosis, and diminished pulse in the extremities. The etiology is believed to be multifactorial resulting from a combination of genetic mutations and defects resulting in altered blood flow in the heart. Several structures can be affected including the left ventricle, aorta, aortic valve, or mitral valve all resulting in decreased systemic blood flow.

Pulmonary atresia is a congenital malformation of the pulmonary valve in which the valve orifice fails to develop. The valve is completely closed thereby obstructing the outflow of blood from the heart to the lungs. The pulmonary valve is located on the right side of the heart between the right ventricle and pulmonary artery. In a normal functioning heart, the opening to the pulmonary valve has three flaps that open and close.

Tricuspid atresia is a form of congenital heart disease whereby there is a complete absence of the tricuspid valve. Therefore, there is an absence of right atrioventricular connection. This leads to a hypoplastic (undersized) or absent right ventricle. This defect is contracted during prenatal development, when the heart does not finish developing. It causes the systemic circulation to be filled with relatively deoxygenated blood. The causes of tricuspid atresia are unknown.
Levo-Transposition of the great arteries is an acyanotic congenital heart defect in which the primary arteries are transposed, with the aorta anterior and to the left of the pulmonary artery; the morphological left and right ventricles with their corresponding atrioventricular valves are also transposed.

The Norwood procedure is the first of three surgeries intended to create a new functional systemic circuit in patients with hypoplastic left heart syndrome and other complex heart defects with single ventricle physiology. The first successful Norwood procedure involving the use of a cardiopulmonary bypass was reported by Dr. William Imon Norwood, Jr. and colleagues in 1981.
Crisscross heart is a type of congenital heart defect where the right atrium is closely associated with the left ventricle in space, and the left atrium is closely associated with the right ventricle.

A double inlet left ventricle (DILV) or "single ventricle", is a congenital heart defect appearing in 5 in 100,000 newborns, where both the left atrium and the right atrium feed into the left ventricle. The right ventricle is hypoplastic or does not exist.
The following outline is provided as an overview of and topical guide to cardiology, the branch of medicine dealing with disorders of the human heart. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease and electrophysiology. Physicians who specialize in cardiology are called cardiologists.

The bidirectional Glenn (BDG) shunt, or bidirectional cavopulmonary anastomosis, is a surgical technique used in pediatric cardiac surgery procedure used to temporarily improve blood oxygenation for patients with a congenital cardiac defect resulting in a single functional ventricle. Creation of a bidirectional shunt reduces the amount of blood volume that the heart needs to pump at the time of surgical repair with the Fontan procedure.

A ventricular outflow tract obstruction is a heart condition in which either the right or left ventricular outflow tract is blocked or obstructed. These obstructions represent a spectrum of disorders. Majority of these cases are congenital, but some are acquired throughout life.
Fetal aortic stenosis is a disorder that occurs when the fetus’ aortic valve does not fully open during development. The aortic valve is a one way valve that is located between the left ventricle and the aorta, keeping blood from leaking back into the ventricle. It has three leaflets that separate when the ventricle contracts to allow blood to move from the ventricle to the aorta. These leaflets come together when the ventricle relaxes.
The Damus–Kaye–Stansel (DKS) procedure is a cardiovascular surgical procedure used as part of the repair of some congenital heart defects. This procedure joins the pulmonary artery and the aorta in situations where the systemic circulation is obstructed. It is commonly used when a patient has the combination of a small left ventricle and a transposition of the great arteries (TGA); in this case, the procedure allows blood to flow from the left ventricle to the aorta.
The Yasui procedure is a pediatric heart operation used to bypass the left ventricular outflow tract (LVOT) that combines the aortic repair of the Norwood procedure and a shunt similar to that used in the Rastelli procedure in a single operation. It is used to repair defects that result in the physiology of hypoplastic left heart syndrome even though both ventricles are functioning normally. These defects are common in DiGeorge syndrome and include interrupted aortic arch and LVOT obstruction (IAA/LVOTO); aortic atresia-severe stenosis with ventricular septal defect (AA/VSD); and aortic atresia with interrupted aortic arch and aortopulmonary window. This procedure allows the surgeon to keep the left ventricle connected to the systemic circulation while using the pulmonary valve as its outflow valve, by connecting them through the ventricular septal defect. The Yasui procedure includes a modified Damus–Kaye–Stansel procedure to connect the aortic and pulmonary roots, allowing the coronary arteries to remain perfused. It was first described in 1987.











