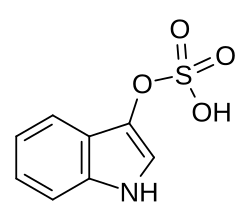
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1H-Indol-3-yl hydrogen sulfate | |
| Other names 3-Indoxylsulfate; 3-Indoxylsulfuric acid; Indol-3-yl sulfate | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C8H7NO4S | |
| Molar mass | 213.21 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Indoxyl sulfate, also known as 3-indoxylsulfate and 3-indoxylsulfuric acid, is a metabolite of dietary L-tryptophan that acts as a cardiotoxin and uremic toxin. [1] [2] [3] High concentrations of indoxyl sulfate in blood plasma are known to be associated with the development and progression of chronic kidney disease and vascular disease in humans. [1] [2] [3] As a uremic toxin, it stimulates glomerular sclerosis and renal interstitial fibrosis. [1] [2]
