Related Research Articles
A protein phosphatase is a phosphatase enzyme that removes a phosphate group from the phosphorylated amino acid residue of its substrate protein. Protein phosphorylation is one of the most common forms of reversible protein posttranslational modification (PTM), with up to 30% of all proteins being phosphorylated at any given time. Protein kinases (PKs) are the effectors of phosphorylation and catalyse the transfer of a γ-phosphate from ATP to specific amino acids on proteins. Several hundred PKs exist in mammals and are classified into distinct super-families. Proteins are phosphorylated predominantly on Ser, Thr and Tyr residues, which account for 79.3, 16.9 and 3.8% respectively of the phosphoproteome, at least in mammals. In contrast, protein phosphatases (PPs) are the primary effectors of dephosphorylation and can be grouped into three main classes based on sequence, structure and catalytic function. The largest class of PPs is the phosphoprotein phosphatase (PPP) family comprising PP1, PP2A, PP2B, PP4, PP5, PP6 and PP7, and the protein phosphatase Mg2+- or Mn2+-dependent (PPM) family, composed primarily of PP2C. The protein Tyr phosphatase (PTP) super-family forms the second group, and the aspartate-based protein phosphatases the third. The protein pseudophosphatases form part of the larger phosphatase family, and in most cases are thought to be catalytically inert, instead functioning as phosphate-binding proteins, integrators of signalling or subcellular traps. Examples of membrane-spanning protein phosphatases containing both active (phosphatase) and inactive (pseudophosphatase) domains linked in tandem are known, conceptually similar to the kinase and pseudokinase domain polypeptide structure of the JAK pseudokinases. A complete comparative analysis of human phosphatases and pseudophosphatases has been completed by Manning and colleagues, forming a companion piece to the ground-breaking analysis of the human kinome, which encodes the complete set of ~536 human protein kinases.

Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.

Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Since heparins depend on the activity of antithrombin, they are considered anticoagulants. Specifically it is also used in the treatment of heart attacks and unstable angina. It is given intravenously or by injection under the skin. Other uses for its anticoagulant properties include inside blood specimen test tubes and kidney dialysis machines.
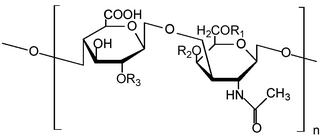
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
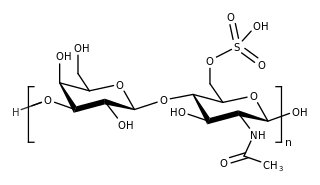
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by Viatris. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.

N-Acetylglucosamine (GlcNAc) is an amide derivative of the monosaccharide glucose. It is a secondary amide between glucosamine and acetic acid. It is significant in several biological systems.
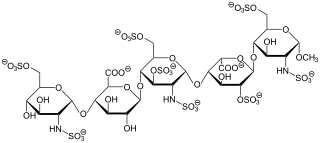
Keratan sulfate (KS), also called keratosulfate, is any of several sulfated glycosaminoglycans that have been found especially in the cornea, cartilage, and bone. It is also synthesized in the central nervous system where it participates both in development and in the glial scar formation following an injury. Keratan sulfates are large, highly hydrated molecules which in joints can act as a cushion to absorb mechanical shock.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.

Uridine diphosphate N-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer N-acetylglucosamine residues to substrates. D-Glucosamine is made naturally in the form of glucosamine-6-phosphate, and is the biochemical precursor of all nitrogen-containing sugars. To be specific, glucosamine-6-phosphate is synthesized from fructose 6-phosphate and glutamine as the first step of the hexosamine biosynthesis pathway. The end-product of this pathway is UDP-GlcNAc, which is then used for making glycosaminoglycans, proteoglycans, and glycolipids.

Pancreatic polypeptide receptor 1, also known as Neuropeptide Y receptor type 4, is a protein that in humans is encoded by the PPYR1 gene.

Protein phosphatase 1 regulatory subunit 1B (PPP1R1B), also known as dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32), is a protein that in humans is encoded by the PPP1R1B gene.

Neurabin-2 is a protein that in humans is encoded by the PPP1R9B gene.

Beta-1,4-galactosyltransferase 7 also known as galactosyltransferase I is an enzyme that in humans is encoded by the B4GALT7 gene. Galactosyltransferase I catalyzes the synthesis of the glycosaminoglycan-protein linkage in proteoglycans. Proteoglycans in turn are structural components of the extracellular matrix that is found between cells in connective tissues.

Carbohydrate sulfotransferase 5 is an enzyme that in humans is encoded by the CHST5 gene.
Glycopeptides are peptides that contain carbohydrate moieties (glycans) covalently attached to the side chains of the amino acid residues that constitute the peptide.

Synapsin I, is the collective name for Synapsin Ia and Synapsin Ib, two nearly identical phosphoproteins that in humans are encoded by the SYN1 gene. In its phosphorylated form, Synapsin I may also be referred to as phosphosynaspin I. Synapsin I is the first of the proteins in the synapsin family of phosphoproteins in the synaptic vesicles present in the central and peripheral nervous systems. Synapsin Ia and Ib are close in length and almost the same in make up, however, Synapsin Ib stops short of the last segment of the C-terminal in the amino acid sequence found in Synapsin Ia.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

Protein O-GlcNAc transferase also known as OGT or O-linked N-acetylglucosaminyltransferase is an enzyme that in humans is encoded by the OGT gene. OGT catalyzes the addition of the O-GlcNAc post-translational modification to proteins.

Protein O-GlcNAcase (EC 3.2.1.169, OGA, glycoside hydrolase O-GlcNAcase, O-GlcNAcase, BtGH84, O-GlcNAc hydrolase) is an enzyme with systematic name (protein)-3-O-(N-acetyl-D-glucosaminyl)-L-serine/threonine N-acetylglucosaminyl hydrolase. OGA is encoded by the OGA gene. This enzyme catalyses the removal of the O-GlcNAc post-translational modification in the following chemical reaction:
- [protein]-3-O-(N-acetyl-β-D-glucosaminyl)-L-serine + H2O ⇌ [protein]-L-serine + N-acetyl-D-glucosamine
- [protein]-3-O-(N-acetyl-β-D-glucosaminyl)-L-threonine + H2O ⇌ [protein]-L-threonine + N-acetyl-D-glucosamine

O-GlcNAc is a reversible enzymatic post-translational modification that is found on serine and threonine residues of nucleocytoplasmic proteins. The modification is characterized by a β-glycosidic bond between the hydroxyl group of serine or threonine side chains and N-acetylglucosamine (GlcNAc). O-GlcNAc differs from other forms of protein glycosylation: (i) O-GlcNAc is not elongated or modified to form more complex glycan structures, (ii) O-GlcNAc is almost exclusively found on nuclear and cytoplasmic proteins rather than membrane proteins and secretory proteins, and (iii) O-GlcNAc is a highly dynamic modification that turns over more rapidly than the proteins which it modifies. O-GlcNAc is conserved across metazoans.
References
- ↑ Finder, Expertise. "Linda Carol Hsieh-Wilson, California Institute of Technology: Learning and memory, Memory and motor control, Neurobiology • Expertise Finder Network". network.expertisefinder.com. Retrieved 2017-05-02.
- ↑ Hsieh-Wilson, L. C.; Schultz, P. G.; Stevens, R. C. (1996-05-28). "Insights into antibody catalysis: structure of an oxygenation catalyst at 1.9-angstrom resolution". Proceedings of the National Academy of Sciences of the United States of America. 93 (11): 5363–5367. Bibcode:1996PNAS...93.5363H. doi: 10.1073/pnas.93.11.5363 . PMC 39251 . PMID 8643580.
- ↑ Hsieh-Wilson, L. C.; Allen, P. B.; Watanabe, T.; Nairn, A. C.; Greengard, P. (1999-04-06). "Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1". Biochemistry. 38 (14): 4365–4373. doi:10.1021/bi982900m. PMID 10194355.
- ↑ Grossman, Stacie D.; Hsieh-Wilson, Linda C.; Allen, Patrick B.; Nairn, Angus C.; Greengard, Paul (2002-01-01). "The actin-binding domain of spinophilin is necessary and sufficient for targeting to dendritic spines". Neuromolecular Medicine. 2 (1): 61–69. doi:10.1385/NMM:2:1:61. PMID 12230305. S2CID 21825701.
- ↑ Hsieh-Wilson, Linda C.; Benfenati, Fabio; Snyder, Gretchen L.; Allen, Patrick B.; Nairn, Angus C.; Greengard, Paul (2003-01-10). "Phosphorylation of spinophilin modulates its interaction with actin filaments". The Journal of Biological Chemistry. 278 (2): 1186–1194. doi: 10.1074/jbc.M205754200 . PMID 12417592. S2CID 11557256.
- ↑ "Linda C. Hsieh-Wilson | www.cce.caltech.edu". www.cce.caltech.edu. Retrieved 2017-05-02.
- ↑ "HHMI Scientist Abstract: Linda C. Hsieh-Wilson, Ph.D." Howard Hughes Medical Institute. Retrieved 2011-07-16.
- ↑ Khidekel, Nelly; Ficarro, Scott B; Clark, Peter M; Bryan, Marian C; Swaney, Danielle L; Rexach, Jessica E; Sun, Yi E; Coon, Joshua J; et al. (2007). "Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics" (PDF). Nature Chemical Biology . 3 (6): 339–348. doi:10.1038/nchembio881. PMID 17496889.
- ↑ Gama, Cristal I; Tully, Sarah E; Sotogaku, Naoki; Clark, Peter M; Rawat, Manish; Vaidehi, Nagarajan; Goddard, William A; Nishi, Akinori; et al. (2006). "Sulfation patterns of glycosaminoglycans encode molecular recognition and activity" (PDF). Nature Chemical Biology. 2 (9): 467–473. doi:10.1038/nchembio810. PMID 16878128. S2CID 1229340.
- ↑ "Sweet Memories of Synapsins?". Science's STKE. 2006 (317): tw472. 2006. doi:10.1126/stke.3172006tw472. S2CID 220299653.
- ↑ Khidekel, Nelly; Ficarro, Scott B.; Peters, Eric C.; Hsieh-Wilson, Linda C. (7 September 2004). "Exploring the O-GlcNAc proteome: Direct identification of O-GlcNAc-modified proteins from the brain". Proceedings of the National Academy of Sciences. 101 (36): 13132–13137. Bibcode:2004PNAS..10113132K. doi: 10.1073/pnas.0403471101 . PMC 516536 . PMID 15340146.
- ↑ http://apps.webofknowledge.com/full_record.do?product=WOS&search_mode=DaisyOneClickSearch&qid=11&SID=1AMO2etzAYAIFJZoUSG&page=1&doc=6&cacheurlFromRightClick=no%5B%5D
- ↑ Hsieh-Wilson, Linda (2013-04-01). "O-GlcNAc Signaling Regulates Cancer Metabolism". The FASEB Journal. 27 (1 Supplement): 452.2. doi: 10.1096/fasebj.27.1_supplement.452.2 .
- ↑ http://apps.webofknowledge.com/full_record.do?product=WOS&search_mode=DaisyOneClickSearch&qid=11&SID=1AMO2etzAYAIFJZoUSG&page=1&doc=21%5B%5D
- ↑ Gama, Cristal I.; Tully, Sarah E.; Sotogaku, Naoki; Clark, Peter M.; Rawat, Manish; Vaidehi, Nagarajan; Goddard, William A.; Nishi, Akinori; Hsieh-Wilson, Linda C. (2006). "Sulfation patterns of glycosaminoglycans encode molecular recognition and activity". Nature Chemical Biology. 2 (9): 467–473. doi:10.1038/nchembio810. PMID 16878128. S2CID 1229340.
- ↑ Tully, Sarah E.; Mabon, Ross; Gama, Cristal I.; Tsai, Sherry M.; Liu, Xuewei; Hsieh-Wilson, Linda C. (2004-06-01). "A Chondroitin Sulfate Small Molecule that Stimulates Neuronal Growth" (PDF). Journal of the American Chemical Society. 126 (25): 7736–7737. doi:10.1021/ja0484045. ISSN 0002-7863. PMID 15212495.
- ↑ Shipp, Eric L.; Hsieh-Wilson, Linda C. (2007-02-01). "Profiling the Sulfation Specificities of Glycosaminoglycan Interactions with Growth Factors and Chemotactic Proteins Using Microarrays". Chemistry & Biology. 14 (2): 195–208. doi: 10.1016/j.chembiol.2006.12.009 . PMID 17317573.
- 1 2 "Web of Science". 2011. Retrieved 2011-07-16.
- ↑ "Linda C. Hsieh-Wilson". Arnold and Mabel Beckman Foundation. Retrieved 1 August 2018.
- ↑ "Arthur C. Cope Scholar Awards". American Chemical Society. Archived from the original on 2011-08-11. Retrieved 2011-07-16.
- ↑ "Top neuroscientists receive awards from IU's Gill Center". IU News Room. Indiana University. Retrieved 2019-09-15.