List of cannabis regulatory agencies may refer to one of the following:
This article includes a list of lists.
| General | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Usage |
| ||||||||
| Variants | |||||||||
| Effects |
| ||||||||
| Culture | |||||||||
| Cannabis rights organizations | |||||||||
| Demographics | |||||||||
| Politics |
| ||||||||
| Related | |||||||||
List of cannabis regulatory agencies may refer to one of the following:

Tincture of cannabis, sometimes known as green dragon, is an alcoholic cannabis concentrate. The solubility of THC in ethanol is greater than 1 g/mL.

The minister of health is the minister of the Crown in the Canadian Cabinet who is responsible for overseeing health-focused government agencies including Health Canada and the Public Health Agency of Canada, as well as enforcing the Canada Health Act, the law governing Canada's universal health care system. The current minister is Jean-Yves Duclos.
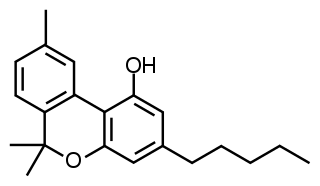
Cannabinol (CBN) is a non-psychoactive cannabinoid found in trace amounts from Cannabis. CBN is mostly found in cannabis that is aged and stored, and is derived from the plant's main psychoactive chemical, tetrahydrocannabinol (THC).

Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. As of 2019, clinical research on CBD included studies related to anxiety, cognition, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions.

The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.

In the United States, the removal of cannabis from Schedule I of the Controlled Substances Act, the most tightly restricted category reserved for drugs that have "no currently accepted medical use,” has been proposed repeatedly since 1972.

The Oregon Liquor and Cannabis Commission (OLCC), formerly known as Oregon Liquor Control Commission is a government agency of the U.S. state of Oregon. The OLCC was created by an act of the Oregon Legislative Assembly in 1933, days after the repeal of prohibition, as a means of providing control over the distribution, sales and consumption of alcoholic beverages. To this end, the agency was given the authority to regulate and license those who manufacture, sell or serve alcohol. Oregon is one of 18 alcoholic beverage control states that directly control the sales of alcoholic beverages in the United States. In 2014, the passage of Oregon Ballot Measure 91 (2014) legalized the recreational use of marijuana in Oregon and gave regulatory authority to the OLCC.
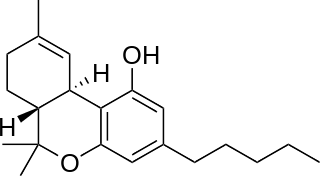
Nabiximols is a specific Cannabis extract that was approved in 2010 as a botanical drug in the United Kingdom. Nabiximols is sold as a mouth spray intended to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms of multiple sclerosis; it was developed by the UK company GW Pharmaceuticals. In 2019 it was proposed that following application of the spray, nabiximols is washed away from the oral mucosa by the saliva flow and ingested into the stomach, with subsequent absorption from the gastro-intestinal tract. Nabiximols is a combination drug standardized in composition, formulation, and dose. Its principal active cannabinoid components are the cannabinoids: tetrahydrocannabinol (THC) and cannabidiol (CBD). Each spray delivers a dose of 2.7 mg THC and 2.5 mg CBD.
A regulatory agency is a government authority that is responsible for exercising autonomous dominion over some area of human activity in a licensing and regulating capacity.
Expanded access or compassionate use is the use of an unapproved drug or medical device under special forms of investigational new drug applications (IND) or IDE application for devices, outside of a clinical trial, by people with serious or life-threatening conditions who do not meet the enrollment criteria for the clinical trial in progress.
Michigan has a republican form of government with three branches of government: the executive branch consisting of the Governor of Michigan and the other independently elected constitutional officers; the legislative branch consisting of the House of Representatives and Senate; and the judicial branch consisting of the one court of justice. The state also allows direct participation of the electorate by initiative, referendum, recall, and ratification.

The Adult Use of Marijuana Act (AUMA) was a 2016 voter initiative to legalize cannabis in California. The full name is the Control, Regulate and Tax Adult Use of Marijuana Act. The initiative passed with 57% voter approval and became law on November 9, 2016, leading to recreational cannabis sales in California by January 2018.

The Michigan Regulation and Taxation of Marijuana Act, also known as Proposal 1, was an initiative that appeared on the November 2018 ballot to legalize cannabis in the U.S. state of Michigan. The initiative allows adults 21 and older to possess up to 2.5 ounces (71 g) of cannabis and to grow up to 12 plants at home. The initiative was approved with 56% of the vote.
The Washington State Department of Agriculture (WSDA) is a cabinet-level agency in the government of Washington which regulates, advocates, and provides services for the state's agricultural industry. The agency was established in 1913 and is headquartered in Olympia, Washington. The current director of the WSDA is Derek Sandison.
The Health Products Regulatory Authority is an Irish regulatory agency. It advises the Irish government. It is responsible for both public and animal health. It regulates medicines, medical devices, clinical trials and other health products and monitors the safety of cosmetics. Until July 2014 it was known as the Irish Medicines Board.
The Office of Cannabis Management is a state agency established upon passage of the Marijuana Regulation and Taxation Act (MRTA) to implement a regulatory framework for medical and adult-use cannabis in the state of New York, along with hemp regulations as well. It was announced by Governor Andrew Cuomo in the January 2019 State of the State address. The office is charged with the regulation and taxation of the cannabis industry in the State of New York, following the legalization of recreational cannabis which was signed into law by Governor Cuomo on March 31, 2021. Tax revenue taken in by the agency was estimated by the Governor to start at $83 million in 2021 and rise to $300 million at full implementation in 2023.
The New Jersey Cannabis Regulatory Commission is a state government agency that regulates the sale of medicinal and recreational marijuana products in New Jersey.