Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.

Cannabis sativa is an annual herbaceous flowering plant. The species was first classified by Carl Linnaeus in 1753. The specific epithet sativa means 'cultivated'. Indigenous to Eastern Asia, the plant is now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history and used as a source of industrial fiber, seed oil, food, and medicine. It is also used as a recreation drug and for religious and spiritual purposes.
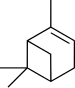
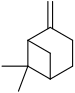
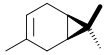
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers. Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca) and big sagebrush.

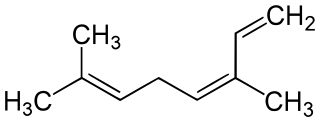
Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature.

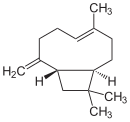
Caryophyllene, more formally (−)-β-caryophyllene (BCP), is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
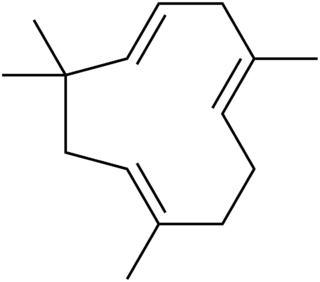
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.
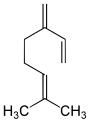
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is cis-3,7-dimethyl-1,3,7-octatriene. β-Ocimene is trans-3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, cis and trans, with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air. Like other terpenes, the ocimenes are nearly insoluble in water, but soluble in common organic solvents.
The terpinenes are a group of isomeric hydrocarbons that are classified as monoterpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source but has been prepared from sabinene. γ-Terpinene and δ-terpinene have been isolated from a variety of plant sources. They are all colorless liquids with a turpentine-like odor.
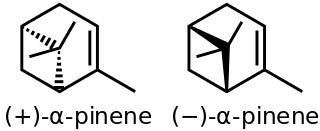
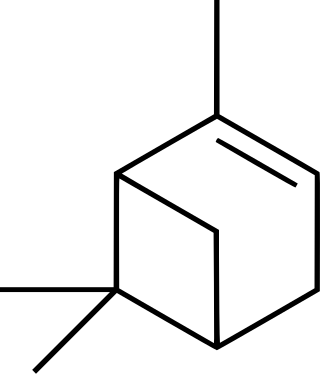
α-Pinene is an organic compound of the terpene class. It is one of the two isomers of pinene, the other being β-pinene. An alkene, it contains a reactive four-membered ring. It is found in the oils of many species of many coniferous trees, notably the Pinus and Picea species. It is also found in the essential oil of rosemary and Satureja myrtifolia. Both enantiomers are known in nature; (1S,5S)- or (−)-α-pinene is more common in European pines, whereas the (1R,5R)- or (+)-α-isomer is more common in North America. The enantiomers' racemic mixture is present in some oils such as eucalyptus oil and orange peel oil.

β-Pinene is a monoterpene, an organic compound found in plants. It is one of the two isomers of pinene, the other being α-pinene. It is colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.

Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. A recent study conducted in the Cosmics Leaving Outdoor Droplets large cloud chamber at CERN, has identified sesquiterpenes—gaseous hydrocarbons that are released by plants—as potentially playing a major role in cloud formation in relatively pristine regions of the atmosphere.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.
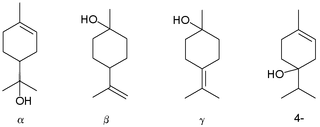
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil. Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. β- and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent.

Cryptocarya agathophylla is a member of the laurel family, Lauraceae, and originates in Madagascar.

Cannabigerol (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis. Cannabigerol is the decarboxylated form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized.

Hash oil or cannabis oil is an oleoresin obtained by the extraction of cannabis or hashish. It is a cannabis concentrate containing many of its resins and terpenes – in particular, tetrahydrocannabinol (THC), cannabidiol (CBD), and other cannabinoids. Hash oil is usually consumed by smoking, vaporizing or eating. Preparations of hash oil may be solid or semi-liquid colloids depending on both production method and temperature and are usually identified by their appearance or characteristics. Color most commonly ranges from transparent golden or light brown, to tan or black. There are various extraction methods, most involving a solvent, such as butane or ethanol.
The entourage effect is a hypothesis that cannabis compounds other than tetrahydrocannabinol (THC) act synergistically with it to modulate the overall psychoactive effects of the plant.
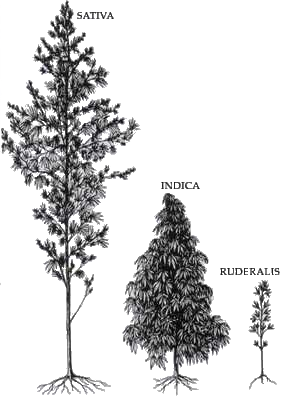
The following outline is provided as an overview of and topical guide to the plant Cannabis sativa and its relatives Cannabis indica and Cannabis ruderalis, the drug cannabis (drug) and the industrial product hemp.
Manuka oil is an essential oil obtained from the steam distillation of the leaves and small branches of the tree Leptospermum scoparium . Though it is used in a wide range of cosmetics, cosmeceuticals and naturopathic and topical medications, manuka oil is a relatively new development; it was first identified during the 1970s and has been produced commercially since the 1980s and investigated by global research teams since then.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.