
The lymphatic system, or lymphoid system, is an organ system in vertebrates that is part of the immune system, and complementary to the circulatory system. It consists of a large network of lymphatic vessels, lymph nodes, lymphoid organs, lymphoid tissues and lymph. Lymph is a clear fluid carried by the lymphatic vessels back to the heart for re-circulation..
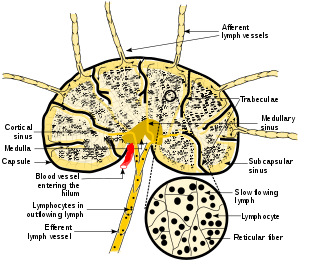
A lymph node, or lymph gland, is a kidney-shaped organ of the lymphatic system and the adaptive immune system. A large number of lymph nodes are linked throughout the body by the lymphatic vessels. They are major sites of lymphocytes that include B and T cells. Lymph nodes are important for the proper functioning of the immune system, acting as filters for foreign particles including cancer cells, but have no detoxification function.

In anatomy, the meninges are the three membranes that envelop the brain and spinal cord. In mammals, the meninges are the dura mater, the arachnoid mater, and the pia mater. Cerebrospinal fluid is located in the subarachnoid space between the arachnoid mater and the pia mater. The primary function of the meninges is to protect the central nervous system.
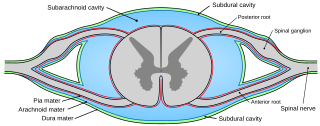
Pia mater, often referred to as simply the pia, is the delicate innermost layer of the meninges, the membranes surrounding the brain and spinal cord. Pia mater is medieval Latin meaning "tender mother". The other two meningeal membranes are the dura mater and the arachnoid mater. Both the pia and arachnoid mater are derivatives of the neural crest while the dura is derived from embryonic mesoderm. The pia mater is a thin fibrous tissue that is permeable to water and small solutes. The pia mater allows blood vessels to pass through and nourish the brain. The perivascular space between blood vessels and pia mater is proposed to be part of a pseudolymphatic system for the brain. When the pia mater becomes irritated and inflamed the result is meningitis.
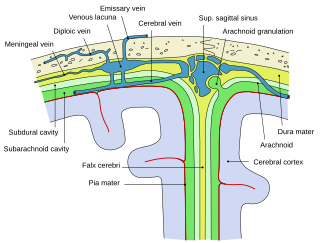
Arachnoid granulations are small protrusions of the arachnoid mater into the outer membrane of the dura mater. They protrude into the dural venous sinuses of the brain, and allow cerebrospinal fluid (CSF) to exit the subarachnoid space and enter the blood stream.

In neuroanatomy, dura mater is a thick membrane made of dense irregular connective tissue that surrounds the brain and spinal cord. It is the outermost of the three layers of membrane called the meninges that protect the central nervous system. The other two meningeal layers are the arachnoid mater and the pia mater. It envelops the arachnoid mater, which is responsible for keeping in the cerebrospinal fluid. It is derived primarily from the neural crest cell population, with postnatal contributions of the paraxial mesoderm.
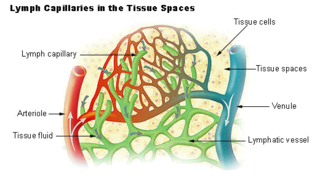
The lymphatic vessels are thin-walled vessels (tubes), structured like blood vessels, that carry lymph. As part of the lymphatic system, lymph vessels are complementary to the cardiovascular system. Lymph vessels are lined by endothelial cells, and have a thin layer of smooth muscle, and adventitia that binds the lymph vessels to the surrounding tissue. Lymph vessels are devoted to the propulsion of the lymph from the lymph capillaries, which are mainly concerned with the absorption of interstitial fluid from the tissues. Lymph capillaries are slightly bigger than their counterpart capillaries of the vascular system. Lymph vessels that carry lymph to a lymph node are called afferent lymph vessels, and those that carry it from a lymph node are called efferent lymph vessels, from where the lymph may travel to another lymph node, may be returned to a vein, or may travel to a larger lymph duct. Lymph ducts drain the lymph into one of the subclavian veins and thus return it to general circulation.

In anatomy, the epidural space is the potential space between the dura mater and vertebrae (spine).

The falx cerebri is a large, crescent-shaped fold of dura mater that descends vertically into the longitudinal fissure between the cerebral hemispheres of the human brain, separating the two hemispheres and supporting dural sinuses that provide venous and CSF drainage to the brain. It is attached to the crista galli anteriorly, and blends with the tentorium cerebelli posteriorly.
The interstitium is a contiguous fluid-filled space existing between a structural barrier, such as a cell membrane or the skin, and internal structures, such as organs, including muscles and the circulatory system. The fluid in this space is called interstitial fluid, comprises water and solutes, and drains into the lymph system. The interstitial compartment is composed of connective and supporting tissues within the body – called the extracellular matrix – that are situated outside the blood and lymphatic vessels and the parenchyma of organs.

Schlemm's canal is a circular lymphatic-like vessel in the eye. It collects aqueous humor from the anterior chamber and delivers it into the episcleral blood vessels. Canaloplasty may be used to widen it.

The dural venous sinuses are venous sinuses (channels) found between the endosteal and meningeal layers of dura mater in the brain. They receive blood from the cerebral veins, and cerebrospinal fluid (CSF) from the subarachnoid space via arachnoid granulations. They mainly empty into the internal jugular vein.

The deep cervical lymph nodes are a group of cervical lymph nodes in the neck that form a chain along the internal jugular vein within the carotid sheath.
Certain sites of the mammalian body have immune privilege, meaning they are able to tolerate the introduction of antigens without eliciting an inflammatory immune response. Tissue grafts are normally recognised as foreign antigens by the body and attacked by the immune system. However, in immune privileged sites, tissue grafts can survive for extended periods of time without rejection occurring. Immunologically privileged sites include:

Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), also known as extracellular link domain containing 1 (XLKD1) is a Link domain-containing hyaladherin, a protein capable of binding to hyaluronic acid (HA), homologous to CD44, the main HA receptor. In humans it is encoded by the LYVE1 gene.
The Lymphatic Endothelium refers to a specialized subset of endothelial cells located in the sinus systems of draining lymph nodes. Specifically, these endothelial cells line the branched sinus systems formed by afferent lymphatic vessels, forming a single-cell layer which functions in a variety of critical physiological processes. These lymphatic endothelial cells contribute directly to immune function and response modulation, provide transport selectivity, and demonstrate orchestration of bidirectional signaling cascades. Additionally, lymphatic endothelial cells may be implicated in downstream immune cell development as well as lymphatic organogenesis. Until recently, lymphatic endothelial cells have not been characterized to their optimal potential. This system is very important in the function of continuous removal of interstitial fluid and proteins, while also having a significant function of entry for leukocytes and tumor cells. This leads to further research that is being developed on the relationship between lymphatic endothelium and metastasis of tumor cells . The lymphatic capillaries are described to be blind ended vessels, and they are made up of a single non-fenestrated layer of endothelial cells; The lymph capillaries function to aid in the uptake of fluids, macromolecules, and cells. Although they are generally similar to blood capillaries, the lymph capillaries have distinct structural differences. Lymph capillaries consist of a more wide and irregular lumen, and the endothelium in lymph capillaries is much thinner as well. Their origin has been speculated to vary based on them being dependent on specific tissue environments, and powered by organ-specific signals.(L. Gutierrez-Miranda, K. Yaniv, 2020). A lymph capillary endothelial cell is distinct from other endothelial cells in that collagen fibers are directly attached to its plasma membrane.
Lymph sacs are a part of the development of the lymphatic system, known as lymphangiogenesis. The lymph sacs are precursors of the lymph vessels. These sacs develop through the processes of vasculogenesis and angiogenesis. However, there is evidence of both of these processes in different organisms. In mice, it is thought that the lymphatic components form through an angiogenic process. But, there is evidence from bird embryos that gives rise to the idea that lymphatic vessels arise in the embryos through a vasculogenesis-like process from the lymphangioblastic endothelial precursor cells.

The glymphatic system is a system for waste clearance in the central nervous system (CNS) of vertebrates. According to this model, cerebrospinal fluid (CSF) flows into the paravascular space around cerebral arteries, combining with interstitial fluid (ISF) and parenchymal solutes, and exiting down venous paravascular spaces. The pathway consists of a para-arterial influx route for CSF to enter the brain parenchyma, coupled to a clearance mechanism for the removal of interstitial fluid (ISF) and extracellular solutes from the interstitial compartments of the brain and spinal cord. Exchange of solutes between CSF and ISF is driven primarily by arterial pulsation and regulated during sleep by the expansion and contraction of brain extracellular space. Clearance of soluble proteins, waste products, and excess extracellular fluid is accomplished through convective bulk flow of ISF, facilitated by astrocytic aquaporin 4 (AQP4) water channels.

Jonathan Kipnis is a neuroscientist, immunologist, and professor of pathology and immunology at the Washington University School of Medicine. His lab studies interactions between the immune system and nervous system. He is best known for his lab's discovery of meningeal lymphatic vessels in humans and mice, which has impacted research on neurodegenerative diseases such as Alzheimer's disease and multiple sclerosis, neuropsychiatric disorders, such as anxiety, and neurodevelopmental disorders such as autism and Rett syndrome.
The subarachnoid lymphatic-like membrane (SLYM) is a highly debated anatomical structure in the human brain that was proposed in 2023 as a possible fourth layer of the meninges.

















