
Pyruvate dehydrogenase complex (PDC) is a complex of three enzymes that converts pyruvate into acetyl-CoA by a process called pyruvate decarboxylation. Acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration, and this complex links the glycolysis metabolic pathway to the citric acid cycle. Pyruvate decarboxylation is also known as the "pyruvate dehydrogenase reaction" because it also involves the oxidation of pyruvate.
Cardiolipin is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. It can also be found in the membranes of most bacteria. The name "cardiolipin" is derived from the fact that it was first found in animal hearts. It was first isolated from the beef heart in the early 1940s by Mary C. Pangborn. In mammalian cells, but also in plant cells, cardiolipin (CL) is found almost exclusively in the inner mitochondrial membrane, where it is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism.
In biochemistry and metabolism, beta oxidation (also β-oxidation) is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA. Acetyl-CoA enters the citric acid cycle, generating NADH and FADH2, which are electron carriers used in the electron transport chain. It is named as such because the beta carbon of the fatty acid chain undergoes oxidation and is converted to a carbonyl group to start the cycle all over again. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.

Malonyl-CoA is a coenzyme A derivative of malonic acid.

Mitochondrial trifunctional protein deficiency is an autosomal recessive fatty acid oxidation disorder that prevents the body from converting certain fats to energy, particularly during periods without food. People with this disorder have inadequate levels of an enzyme that breaks down a certain group of fats called long-chain fatty acids.

Very long-chain specific acyl-CoA dehydrogenase, mitochondrial (VLCAD) is an enzyme that in humans is encoded by the ACADVL gene.

Tafazzin is a protein that in humans is encoded by the TAFAZZIN gene. Tafazzin is highly expressed in cardiac and skeletal muscle, and functions as a phospholipid-lysophospholipid transacylase. It catalyzes remodeling of immature cardiolipin to its mature composition containing a predominance of tetralinoleoyl moieties. Several different isoforms of the tafazzin protein are produced from the TAFAZZIN gene. A long form and a short form of each of these isoforms is produced; the short form lacks a hydrophobic leader sequence and may exist as a cytoplasmic protein rather than being membrane-bound. Other alternatively spliced transcripts have been described but the full-length nature of all these transcripts is not known. Most isoforms are found in all tissues, but some are found only in certain types of cells. Mutations in the TAFAZZIN gene have been associated with mitochondrial deficiency, Barth syndrome, dilated cardiomyopathy (DCM), hypertrophic DCM, endocardial fibroelastosis, left ventricular noncompaction (LVNC), breast cancer, papillary thyroid carcinoma, non-small cell lung cancer, glioma, gastric cancer, thyroid neoplasms, and rectal cancer.

ACADM is a gene that provides instructions for making an enzyme called acyl-coenzyme A dehydrogenase that is important for breaking down (degrading) a certain group of fats called medium-chain fatty acids.
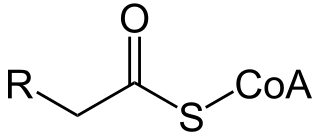
Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the universal biochemical energy carrier.
Fatty acid degradation is the process in which fatty acids are broken down into their metabolites, in the end generating acetyl-CoA, the entry molecule for the citric acid cycle, the main energy supply of living organisms, including bacteria and animals. It includes three major steps:

Trifunctional enzyme subunit alpha, mitochondrial also known as hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase, alpha subunit is a protein that in humans is encoded by the HADHA gene. Mutations in HADHA have been associated with trifunctional protein deficiency or long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency.

Carnitine palmitoyltransferase I (CPT1) also known as carnitine acyltransferase I, CPTI, CAT1, CoA:carnitine acyl transferase (CCAT), or palmitoylCoA transferase I, is a mitochondrial enzyme responsible for the formation of acyl carnitines by catalyzing the transfer of the acyl group of a long-chain fatty acyl-CoA from coenzyme A to l-carnitine. The product is often Palmitoylcarnitine, but other fatty acids may also be substrates. It is part of a family of enzymes called carnitine acyltransferases. This "preparation" allows for subsequent movement of the acyl carnitine from the cytosol into the intermembrane space of mitochondria.

Trifunctional enzyme subunit beta, mitochondrial (TP-beta) also known as 3-ketoacyl-CoA thiolase, acetyl-CoA acyltransferase, or beta-ketothiolase is an enzyme that in humans is encoded by the HADHB gene.

Acetyl-CoA acetyltransferase, mitochondrial, also known as acetoacetyl-CoA thiolase, is an enzyme that in humans is encoded by the ACAT1 gene.

Modern biological research has revealed strong evidence that the enzymes of the mitochondrial respiratory chain assemble into larger, supramolecular structures called supercomplexes, instead of the traditional fluid model of discrete enzymes dispersed in the inner mitochondrial membrane. These supercomplexes are functionally active and necessary for forming stable respiratory complexes.
Monolysocardiolipin (MLCL) is a phospholipid with three fatty acid chains located in the inner membrane of mitochondria.
Monolysocardiolipin acyltransferase is a mitochondrial acyltransferase that facilitates the remodeling of monolysocardiolipin (MLCL) into cardiolipin.

Acyl-CoA thioesterase 9 is a protein that is encoded by the human ACOT9 gene. It is a member of the acyl-CoA thioesterase superfamily, which is a group of enzymes that hydrolyze Coenzyme A esters. There is no known function, however it has been shown to act as a long-chain thioesterase at low concentrations, and a short-chain thioesterase at high concentrations.
Acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT1) is a polyglycerophospholipid acyltransferase of the endoplasmic reticulum which is primarily known for catalyzing the acylation of monolysocardiolipin back into cardiolipin, although it does catalyze the acylation of other polyglycerophospholipids.

Perilipin 5, also known as Oxpatperilipin 5 or PLIN5, is a protein that belongs to perilipin family. This protein group has been shown to be responsible for lipid droplet's biogenesis, structure and degradation. In particular, Perilipin 5 is a lipid droplet-associated protein whose function is to keep the balance between lipolysis and lipogenesis, as well as maintaining lipid droplet homeostasis. For example, in oxidative tissues, muscular tissues and cardiac tissues, PLIN5 promotes association between lipid droplets and mitochondria.













