
The International Bureau of Weights and Measures is an intergovernmental organisation, through which its 59 member-states act on measurement standards in four areas: chemistry, ionising radiation, physical metrology, as well as Coordinated Universal Time. It is based in Saint-Cloud, near Paris, France. The organisation has been referred to as IBWM in older literature.
The General Conference on Weights and Measures is the supreme authority of the International Bureau of Weights and Measures (BIPM), the intergovernmental organization established in 1875 under the terms of the Metre Convention through which member states act together on matters related to measurement science and measurement standards. The CGPM is made up of delegates of the governments of the member states and observers from the Associates of the CGPM. It elects the International Committee for Weights and Measures as the supervisory board of the BIPM to direct and supervise it.

The kilogram is the base unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially. It means 'one thousand grams'.

The litre or liter is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metre (m3). A cubic decimetre occupies a volume of 10 cm × 10 cm × 10 cm and is thus equal to one-thousandth of a cubic metre.

The metre is the base unit of length in the International System of Units (SI).

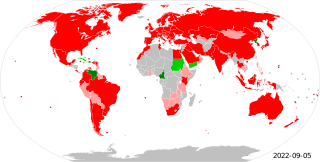
Metrication or metrification is the act or process of converting to the metric system of measurement. All over the world, countries have transitioned from local and traditional units of measurement to the metric system. This process began in France during the 1790s, and is still continuing more than 200 years later, with the modern International System of Units, as the metric system has not been fully adopted in all countries and areas.
The Metre Convention, also known as the Treaty of the Metre, is an international treaty that was signed in Paris on 20 May 1875 by representatives of 17 nations: Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, Germany, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States of America, and Venezuela.
The International System of Units, internationally known by the abbreviation SI, is the modern form of the metric system and the world's most widely used system of measurement. Established and maintained by the General Conference on Weights and Measures (CGPM), it is the only system of measurement with an official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce.

The SI base units are the standard units of measurement defined by the International System of Units (SI) for the seven base quantities of what is now known as the International System of Quantities: they are notably a basic set from which all other SI units can be derived. The units and their physical quantities are the second for time, the metre for length or distance, the kilogram for mass, the ampere for electric current, the kelvin for thermodynamic temperature, the mole for amount of substance, and the candela for luminous intensity. The SI base units are a fundamental part of modern metrology, and thus part of the foundation of modern science and technology.
A metric prefix is a unit prefix that precedes a basic unit of measure to indicate a multiple or submultiple of the unit. All metric prefixes used today are decadic. Each prefix has a unique symbol that is prepended to any unit symbol. The prefix kilo-, for example, may be added to gram to indicate multiplication by one thousand: one kilogram is equal to one thousand grams. The prefix milli-, likewise, may be added to metre to indicate division by one thousand; one millimetre is equal to one thousandth of a metre.

The metric system is a system of measurement that is a decimal system. The current international standard for the metric system is the International System of Units, in which all units can be expressed in terms of seven base units. The units that serves as the SI base units are the metre, kilogram, second, ampere, kelvin, mole, and candel.

Metrology is the scientific study of measurement. It establishes a common understanding of units, crucial in linking human activities. Modern metrology has its roots in the French Revolution's political motivation to standardise units in France when a length standard taken from a natural source was proposed. This led to the creation of the decimal-based metric system in 1795, establishing a set of standards for other types of measurements. Several other countries adopted the metric system between 1795 and 1875; to ensure conformity between the countries, the Bureau International des Poids et Mesures (BIPM) was established by the Metre Convention. This has evolved into the International System of Units (SI) as a result of a resolution at the 11th General Conference on Weights and Measures (CGPM) in 1960.

The gram is a unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram.

France has a unique history of units of measurement due to its radical decision to invent and adopt the metric system after the French Revolution.

The CSIR- National Physical Laboratory of India, situated in New Delhi, is the measurement standards laboratory of India. It maintains standards of SI units in India and calibrates the national standards of weights and measures.

The degree Celsius is the unit of temperature on the Celsius scale, one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The degree Celsius can refer to a specific temperature on the Celsius scale or a unit to indicate a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who developed a variant of it in 1742. The unit was called centigrade in several languages for many years. In 1948, the International Committee for Weights and Measures renamed it to honor Celsius and also to remove confusion with the term for one hundredth of a gradian in some languages. Most countries use this scale; the other major scale, Fahrenheit, is still used in the United States, some island territories, and Liberia. The Kelvin scale is of use in the sciences, with 0 K (−273.15 °C) representing absolute zero.

The kelvin, symbol K, is a unit of measurement for temperature. The Kelvin scale is an absolute scale, which is defined such that 0 K is absolute zero and a change of thermodynamic temperature T by 1 kelvin corresponds to a change of thermal energy kT by 1.380649×10−23 J. The Boltzmann constant k = 1.380649×10−23 J⋅K−1 was exactly defined in the 2019 redefinition of the SI base units such that the triple point of water is 273.16±0.0001 K. The kelvin is the base unit of temperature in the International System of Units (SI), used alongside its prefixed forms. It is named after the Belfast-born and University of Glasgow-based engineer and physicist William Thomson, 1st Baron Kelvin (1824–1907).

In metrology, a standard is an object, system, or experiment that bears a defined relationship to a unit of measurement of a physical quantity. Standards are the fundamental reference for a system of weights and measures, against which all other measuring devices are compared. Historical standards for length, volume, and mass were defined by many different authorities, which resulted in confusion and inaccuracy of measurements. Modern measurements are defined in relationship to internationally standardized reference objects, which are used under carefully controlled laboratory conditions to define the units of length, mass, electrical potential, and other physical quantities.

In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artifacts such as the standard kilogram. Effective 20 May 2019, the 144th anniversary of the Metre Convention, the kilogram, ampere, kelvin, and mole are now defined by setting exact numerical values, when expressed in SI units, for the Planck constant, the elementary electric charge, the Boltzmann constant, and the Avogadro constant, respectively. The second, metre, and candela had previously been redefined using physical constants. The four new definitions aimed to improve the SI without changing the value of any units, ensuring continuity with existing measurements. In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes, which the International Committee for Weights and Measures (CIPM) had proposed earlier that year after determining that previously agreed conditions for the change had been met. These conditions were satisfied by a series of experiments that measured the constants to high accuracy relative to the old SI definitions, and were the culmination of decades of research.

The history of the metric system began during the Age of Enlightenment with measures of length and weight derived from nature, along with their decimal multiples and fractions. The system became the standard of France and Europe within half a century. Other measures with unity ratios were added, and the system went on to be adopted across the world.

















