
The rhinovirus is a positive-sense, single-stranded RNA virus belonging to the genus Enterovirus in the family Picornaviridae. Rhinovirus is the most common viral infectious agent in humans and is the predominant cause of the common cold.

Orthohantavirus is a genus of single-stranded, enveloped, negative-sense RNA viruses in the family Hantaviridae within the order Bunyavirales. Members of this genus may be called orthohantaviruses or simply hantaviruses.

Picornaviruses are a group of related nonenveloped RNA viruses which infect vertebrates including fish, mammals, and birds. They are viruses that represent a large family of small, positive-sense, single-stranded RNA viruses with a 30 nm icosahedral capsid. The viruses in this family can cause a range of diseases including the common cold, poliomyelitis, meningitis, hepatitis, and paralysis.

Foot-and-mouth disease virus (FMDV) is a virus in the genus Aphthovirus that causes foot-and-mouth disease. As a member of the family Picornaviridae, FMDV is a positive-sense, single-stranded RNA virus. Like other members of the Picornavirus family, FMDV is small and unenveloped, with an icosahedral capsid.
Polyomaviridae is a family of viruses whose natural hosts are primarily mammals and birds. As of 2024, there are eight recognized genera. 14 species are known to infect humans, while others, such as Simian Virus 40, have been identified in humans to a lesser extent. Most of these viruses are very common and typically asymptomatic in most human populations studied. BK virus is associated with nephropathy in renal transplant and non-renal solid organ transplant patients, JC virus with progressive multifocal leukoencephalopathy, and Merkel cell virus with Merkel cell cancer.

Rubella virus (RuV) is the pathogenic agent of the disease rubella, transmitted only between humans via the respiratory route, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy.
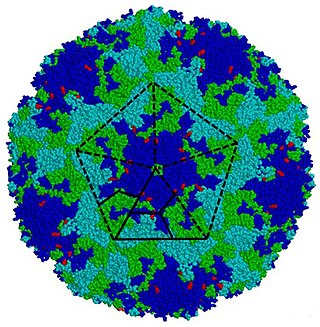
Enterovirus is a genus of positive-sense single-stranded RNA viruses associated with several human and mammalian diseases. Enteroviruses are named by their transmission-route through the intestine.
Echovirus is a polyphyletic group of viruses associated with enteric disease in humans. The name is derived from "enteric cytopathic human orphan virus". These viruses were originally not associated with disease, but many have since been identified as disease-causing agents. The term "echovirus" was used in the scientific names of numerous species, but all echoviruses are now recognized as strains of various species, most of which are in the family Picornaviridae.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.

An arenavirus is a bi- or trisegmented ambisense RNA virus that is a member of the family Arenaviridae. These viruses infect rodents and occasionally humans. A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease, mostly in boa constrictors. At least eight arenaviruses are known to cause human disease. The diseases derived from arenaviruses range in severity. Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus. Hemorrhagic fever syndromes, including Lassa fever, are derived from infections such as Guanarito virus, Junin virus, Lassa virus, Lujo virus, Machupo virus, Sabia virus, or Whitewater Arroyo virus. Because of the epidemiological association with rodents, some arenaviruses and bunyaviruses are designated as roboviruses.

Hepacivirus is a genus of positive-strand RNA viruses in the family Flaviviridae. The hepatitis C virus (HCV), in species Hepacivirus C, infects humans and is associated with hepatitis and hepatocellular carcinoma. There are fourteen species in the genus which infect a range of other vertebrate.

Cardiovirus are a group of viruses within order Picornavirales, family Picornaviridae. Vertebrates serve as natural hosts for these viruses.

Murine coronavirus (M-CoV) is a virus in the genus Betacoronavirus that infects mice. Belonging to the subgenus Embecovirus, murine coronavirus strains are enterotropic or polytropic. Enterotropic strains include mouse hepatitis virus (MHV) strains D, Y, RI, and DVIM, whereas polytropic strains, such as JHM and A59, primarily cause hepatitis, enteritis, and encephalitis. Murine coronavirus is an important pathogen in the laboratory mouse and the laboratory rat. It is the most studied coronavirus in animals other than humans, and has been used as an animal disease model for many virological and clinical studies.
Parechovirus B, formerly called the Ljungan virus, was first discovered in the mid-1990s after being isolated from a bank vole near the Ljungan river in Medelpad county, Sweden. It has since been established that Parechovirus B, which is also found in several places in Europe and America, causes serious illness in wild as well as laboratory animals. Several scientific articles have recently reported findings indicating that Parechovirus B is associated with malformations, intrauterine fetal death, and sudden infant death syndrome in humans. In addition, studies are being conducted worldwide to investigate the possible connection of the virus to diabetes, neurological and other illnesses in humans.
Kobuvirus is a genus of viruses in the order Picornavirales, in the family Picornaviridae. Humans and cattle serve as natural hosts. There are six species in this genus. Diseases associated with this genus include: gastroenteritis. The genus was named because of the virus particles' lumpy appearance by electron microscopy; "kobu" means "knob" in Japanese.

The Human Parechovirus 1 cis regulatory element is an RNA element which is located in the 5'-terminal 112 nucleotides of the genome of human parechovirus 1 (HPeV1). The element consists of two stem-loop structures together with a pseudoknot. Disruption of any of these elements impairs both viral replication and growth.
Gyrovirus is a genus of viruses, in the family Anelloviridae. Until 2011, chicken anemia virus was the only Gyrovirus identified, but since then gyroviruses have also been identified in humans. Diseases associated with this genus include: chicken infectious anemia, which is associated with depletion of cortical thymocytes and erythroblastoid cells.

Bottigliavirus is the only genus in the family Ampullaviridae and contains 3 species. Ampullaviridae infect archaea of the genus Acidianus. The name of the family and genus is derived from the Latin word for bottle, ampulla, due to the virions having the shape of a bottle. The family was first described during an investigation of the microbial flora of hot springs in Italy.
Tula orthohantavirus, formerly Tula virus (TULV), is a single-stranded, negative-sense RNA virus species of orthohantavirus first isolated from a European common vole found in Central Russia and primarly carried by rodents. It causes Hantavirus hemorrhagic fever with renal syndrome. The Microtus species are also found in North America, Europe, Scandinavia, Slovenia, Asia, and Western Russia. Human cases of Tula orthohantavirus have also been reported in Switzerland and Germany.
Echovirus 9 is a serotype of echovirus. When first discovered, it was labelled as a coxsackie A virus, A23. It was later discovered that A23 was an echovirus antigenically identical to the already-known echovirus 9.













