Related Research Articles

The pituitary gland is an endocrine gland in vertebrates. In humans, the pituitary gland is located at the base of the brain, protruding off the bottom of the hypothalamus. The human pituitary gland is oval shaped, about the size of a chickpea, and weighs 0.5 grams (0.018 oz) on average.

The hypothalamus is a small part of the brain that contains a number of nuclei with a variety of functions. One of the most important functions is to link the nervous system to the endocrine system via the pituitary gland. The hypothalamus is located below the thalamus and is part of the limbic system. It forms the ventral part of the diencephalon. All vertebrate brains contain a hypothalamus. In humans, it is the size of an almond.

Corticotropin-releasing hormone (CRH) is a peptide hormone involved in stress responses. It is a releasing hormone that belongs to corticotropin-releasing factor family. In humans, it is encoded by the CRH gene. Its main function is the stimulation of the pituitary synthesis of adrenocorticotropic hormone (ACTH), as part of the hypothalamic–pituitary–adrenal axis.
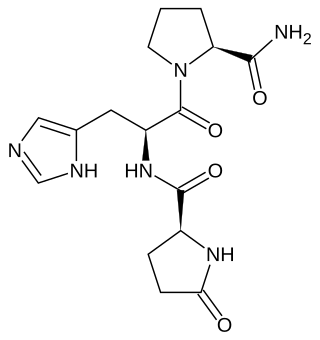
Thyrotropin-releasing hormone (TRH) is a hypophysiotropic hormone produced by neurons in the hypothalamus that stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary.

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.
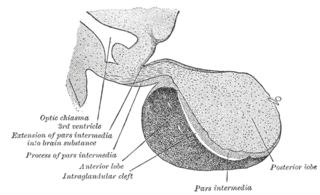
A major organ of the endocrine system, the anterior pituitary is the glandular, anterior lobe that together with the posterior lobe makes up the pituitary gland (hypophysis) which, in humans, is located at the base of the brain, protruding off the bottom of the hypothalamus.
The posterior pituitary is the posterior lobe of the pituitary gland which is part of the endocrine system. The posterior pituitary is not glandular as is the anterior pituitary. Instead, it is largely a collection of axonal projections from the hypothalamus that terminate behind the anterior pituitary, and serve as a site for the secretion of neurohypophysial hormones directly into the blood. The hypothalamic–neurohypophyseal system is composed of the hypothalamus, posterior pituitary, and these axonal projections.

The supraoptic nucleus (SON) is a nucleus of magnocellular neurosecretory cells in the hypothalamus of the mammalian brain. The nucleus is situated at the base of the brain, adjacent to the optic chiasm. In humans, the SON contains about 3,000 neurons.

The paraventricular nucleus is a nucleus in the hypothalamus. Anatomically, it is adjacent to the third ventricle and many of its neurons project to the posterior pituitary. These projecting neurons secrete oxytocin and a smaller amount of vasopressin, otherwise the nucleus also secretes corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH). CRH and TRH are secreted into the hypophyseal portal system and act on different targets neurons in the anterior pituitary. PVN is thought to mediate many diverse functions through these different hormones, including osmoregulation, appetite, and the response of the body to stress.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.
Magnocellular neurosecretory cells are large neuroendocrine cells within the supraoptic nucleus and paraventricular nucleus of the hypothalamus. They are also found in smaller numbers in accessory cell groups between these two nuclei, the largest one being the circular nucleus. There are two types of magnocellular neurosecretory cells, oxytocin-producing cells and vasopressin-producing cells, but a small number can produce both hormones. These cells are neuroendocrine neurons, are electrically excitable, and generate action potentials in response to afferent stimulation. Vasopressin is produced from the vasopressin-producing cells via the AVP gene, a molecular output of circadian pathways.
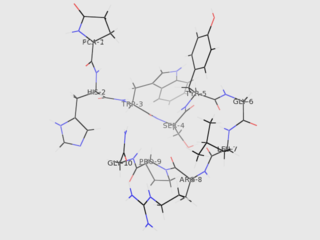
Gonadotropin-releasing hormone (GnRH) is a releasing hormone responsible for the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. GnRH is a tropic peptide hormone synthesized and released from GnRH neurons within the hypothalamus. The peptide belongs to gonadotropin-releasing hormone family. It constitutes the initial step in the hypothalamic–pituitary–gonadal axis.

The arcuate nucleus of the hypothalamus is an aggregation of neurons in the mediobasal hypothalamus, adjacent to the third ventricle and the median eminence. The arcuate nucleus includes several important and diverse populations of neurons that help mediate different neuroendocrine and physiological functions, including neuroendocrine neurons, centrally projecting neurons, and astrocytes. The populations of neurons found in the arcuate nucleus are based on the hormones they secrete or interact with and are responsible for hypothalamic function, such as regulating hormones released from the pituitary gland or secreting their own hormones. Neurons in this region are also responsible for integrating information and providing inputs to other nuclei in the hypothalamus or inputs to areas outside this region of the brain. These neurons, generated from the ventral part of the periventricular epithelium during embryonic development, locate dorsally in the hypothalamus, becoming part of the ventromedial hypothalamic region. The function of the arcuate nucleus relies on its diversity of neurons, but its central role is involved in homeostasis. The arcuate nucleus provides many physiological roles involved in feeding, metabolism, fertility, and cardiovascular regulation.

The median eminence is generally defined as the portion of the ventral hypothalamus from which the portal vessels arise. The median eminence is a small swelling on the tuber cinereum, posterior to and atop the pituitary stalk; it lies in the area roughly bounded on its posterolateral region by the cerebral peduncles, and on its anterolateral region by the optic chiasm.
A neurohormone is any hormone produced and released by neuroendocrine cells into the blood. By definition of being hormones, they are secreted into the circulation for systemic effect, but they can also have a role of neurotransmitter or other roles such as autocrine (self) or paracrine (local) messenger.
Neuroendocrine cells are cells that receive neuronal input and, as a consequence of this input, release messenger molecules (hormones) into the blood. In this way they bring about an integration between the nervous system and the endocrine system, a process known as neuroendocrine integration. An example of a neuroendocrine cell is a cell of the adrenal medulla, which releases adrenaline to the blood. The adrenal medullary cells are controlled by the sympathetic division of the autonomic nervous system. These cells are modified postganglionic neurons. Autonomic nerve fibers lead directly to them from the central nervous system. The adrenal medullary hormones are kept in vesicles much in the same way neurotransmitters are kept in neuronal vesicles. Hormonal effects can last up to ten times longer than those of neurotransmitters. Sympathetic nerve fiber impulses stimulate the release of adrenal medullary hormones. In this way the sympathetic division of the autonomic nervous system and the medullary secretions function together.
Neuroendocrinology is the branch of biology which studies the interaction between the nervous system and the endocrine system; i.e. how the brain regulates the hormonal activity in the body. The nervous and endocrine systems often act together in a process called neuroendocrine integration, to regulate the physiological processes of the human body. Neuroendocrinology arose from the recognition that the brain, especially the hypothalamus, controls secretion of pituitary gland hormones, and has subsequently expanded to investigate numerous interconnections of the endocrine and nervous systems.

The hypophyseal portal system is a system of blood vessels in the microcirculation at the base of the brain, connecting the hypothalamus with the anterior pituitary. Its main function is to quickly transport and exchange hormones between the hypothalamus arcuate nucleus and anterior pituitary gland. The capillaries in the portal system are fenestrated which allows a rapid exchange between the hypothalamus and the pituitary. The main hormones transported by the system include gonadotropin-releasing hormone, corticotropin-releasing hormone, growth hormone–releasing hormone, and thyrotropin-releasing hormone.
The periventricular nucleus is a thin sheet of small neurons located in the wall of the third ventricle, a composite structure of the hypothalamus. It functions in analgesia.

Neurophysin II is a carrier protein with a size of 19,687.3 Da and is made up of a dimer of two virtually identical chains of amino acids. Neurophysin II is a cleavage product of the AVP gene. It is a neurohypophysial hormone that is transported in vesicles with vasopressin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with vasopressin and released with vasopressin into the bloodstream, its biological action is unclear. Neurophysin II is also known as a stimulator of prolactin secretion.
References
- 1 2 3 Hall, John E. (2021). Guyton and Hall Textbook of Medical Physiology. Michael E. Hall (14th ed.). Philadelphia, PA. pp. 931–932. ISBN 978-0-323-59712-8. OCLC 1129099861.
{{cite book}}: CS1 maint: location missing publisher (link) - ↑ Splittgerber, Ryan (2019). Snell's Clinical Neuroanatomy. Richard S. Preceded by Snell (8th ed.). Philadelphia. pp. 379–380. ISBN 978-1-4963-4675-9. OCLC 1045082168.
{{cite book}}: CS1 maint: location missing publisher (link) - ↑ Sawchenko, PE (Dec 29, 1987). "Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus". Brain Research. 437 (2): 253–63. doi:10.1016/0006-8993(87)91641-6. PMID 3325130. S2CID 38822848.
- ↑ Kovács, KJ; Sawchenko, PE (January 1996). "Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons". The Journal of Neuroscience. 16 (1): 262–73. doi: 10.1523/JNEUROSCI.16-01-00262.1996 . PMC 6578740 . PMID 8613792.
- ↑ Ghamari-Langroudi, M.; Vella, K. R.; Srisai, D.; Sugrue, M. L.; Hollenberg, A. N.; Cone, R. D. (13 October 2010). "Regulation of Thyrotropin-Releasing Hormone-Expressing Neurons in Paraventricular Nucleus of the Hypothalamus by Signals of Adiposity". Molecular Endocrinology. 24 (12): 2366–2381. doi:10.1210/me.2010-0203. PMC 2999480 . PMID 20943814.
- ↑ Lennard, DE; Eckert, WA; Merchenthaler, I (April 1993). "Corticotropin-releasing hormone neurons in the paraventricular nucleus project to the external zone of the median eminence: a study combining retrograde labeling with immunocytochemistry". Journal of Neuroendocrinology. 5 (2): 175–81. doi:10.1111/j.1365-2826.1993.tb00378.x. PMID 8485552. S2CID 9640772.
- 1 2 Sawchenko, PE; Swanson, LW; Vale, WW (March 1984). "Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat". Proceedings of the National Academy of Sciences of the United States of America. 81 (6): 1883–7. Bibcode:1984PNAS...81.1883S. doi: 10.1073/pnas.81.6.1883 . PMC 345027 . PMID 6369332.
- ↑ Horn, A. M.; Robinson, I. C. A. F.; Fink, G. (1 February 1985). "Oxytocin and vasopressin in rat hypophysial portal blood: experimental studies in normal and Brattleboro rats". Journal of Endocrinology. 104 (2): 211–NP. doi:10.1677/joe.0.1040211. PMID 3968510.
- ↑ Freeman, ME; Kanyicska, B; Lerant, A; Nagy, G (October 2000). "Prolactin: structure, function, and regulation of secretion". Physiological Reviews. 80 (4): 1523–631. doi:10.1152/physrev.2000.80.4.1523. PMID 11015620.
- ↑ Johnston, CA; Negro-Vilar, A (January 1988). "Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms". Endocrinology. 122 (1): 341–50. doi:10.1210/endo-122-1-341. PMID 3335212.
- ↑ Watanobe, H; Takebe, K (April 1993). "In vivo release of neurotensin from the median eminence of ovariectomized estrogen-primed rats as estimated by push-pull perfusion: correlation with luteinizing hormone and prolactin surges". Neuroendocrinology. 57 (4): 760–4. doi:10.1159/000126434. PMID 8367038.