A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.

The striatum or corpus striatum is a cluster of interconnected nuclei that make up the largest structure of the subcortical basal ganglia. The striatum is a critical component of the motor and reward systems; receives glutamatergic and dopaminergic inputs from different sources; and serves as the primary input to the rest of the basal ganglia.

The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

The basal ganglia (BG) or basal nuclei are a group of subcortical nuclei found in the brains of vertebrates. In humans and other primates, differences exist, primarily in the division of the globus pallidus into external and internal regions, and in the division of the striatum. Positioned at the base of the forebrain and the top of the midbrain, they have strong connections with the cerebral cortex, thalamus, brainstem and other brain areas. The basal ganglia are associated with a variety of functions, including regulating voluntary motor movements, procedural learning, habit formation, conditional learning, eye movements, cognition, and emotion.
The mesolimbic pathway, sometimes referred to as the reward pathway, is a dopaminergic pathway in the brain. The pathway connects the ventral tegmental area in the midbrain to the ventral striatum of the basal ganglia in the forebrain. The ventral striatum includes the nucleus accumbens and the olfactory tubercle.

The nucleus accumbens is a region in the basal forebrain rostral to the preoptic area of the hypothalamus. The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum. The ventral striatum and dorsal striatum collectively form the striatum, which is the main component of the basal ganglia. The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle. Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.
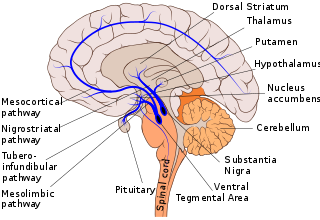
Dopaminergic pathways in the human brain are involved in both physiological and behavioral processes including movement, cognition, executive functions, reward, motivation, and neuroendocrine control. Each pathway is a set of projection neurons, consisting of individual dopaminergic neurons.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.

The ventral tegmental area (VTA), also known as the ventral tegmental area of Tsai, or simply ventral tegmentum, is a group of neurons located close to the midline on the floor of the midbrain. The VTA is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and other dopamine pathways; it is widely implicated in the drug and natural reward circuitry of the brain. The VTA plays an important role in a number of processes, including reward cognition and orgasm, among others, as well as several psychiatric disorders. Neurons in the VTA project to numerous areas of the brain, ranging from the prefrontal cortex to the caudal brainstem and several regions in between.
Motivational salience is a cognitive process and a form of attention that motivates or propels an individual's behavior towards or away from a particular object, perceived event or outcome. Motivational salience regulates the intensity of behaviors that facilitate the attainment of a particular goal, the amount of time and energy that an individual is willing to expend to attain a particular goal, and the amount of risk that an individual is willing to accept while working to attain a particular goal.

The habenula is a small bilateral neuronal structure in the brain of vertebrates, that has also been called a microstructure since it is no bigger than a pea. The naming as little rein describes its elongated shape in the epithalamus, where it borders the third ventricle, and lies in front of the pineal gland.

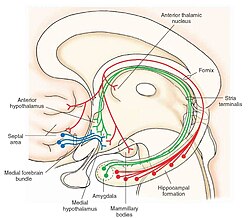
The septal area, consisting of the lateral septum and medial septum, is an area in the lower, posterior part of the medial surface of the frontal lobe, and refers to the nearby septum pellucidum.

The olfactory tubercle (OT), also known as the tuberculum olfactorium, is a multi-sensory processing center that is contained within the olfactory cortex and ventral striatum and plays a role in reward cognition. The OT has also been shown to play a role in locomotor and attentional behaviors, particularly in relation to social and sensory responsiveness, and it may be necessary for behavioral flexibility. The OT is interconnected with numerous brain regions, especially the sensory, arousal, and reward centers, thus making it a potentially critical interface between processing of sensory information and the subsequent behavioral responses.

Medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), are a special type of inhibitory GABAergic neuron representing approximately 90% of neurons within the human striatum, a basal ganglia structure. Medium spiny neurons have two primary phenotypes : D1-type MSNs of the direct pathway and D2-type MSNs of the indirect pathway. Most striatal MSNs contain only D1-type or D2-type dopamine receptors, but a subpopulation of MSNs exhibit both phenotypes.
Brain stimulation reward (BSR) is a pleasurable phenomenon elicited via direct stimulation of specific brain regions, originally discovered by James Olds and Peter Milner. BSR can serve as a robust operant reinforcer. Targeted stimulation activates the reward system circuitry and establishes response habits similar to those established by natural rewards, such as food and sex. Experiments on BSR soon demonstrated that stimulation of the lateral hypothalamus, along with other regions of the brain associated with natural reward, was both rewarding as well as motivation-inducing. Electrical brain stimulation and intracranial drug injections produce robust reward sensation due to a relatively direct activation of the reward circuitry. This activation is considered to be more direct than rewards produced by natural stimuli, as those signals generally travel through the more indirect peripheral nerves. BSR has been found in all vertebrates tested, including humans, and it has provided a useful tool for understanding how natural rewards are processed by specific brain regions and circuits, as well the neurotransmission associated with the reward system.

The reward system is a group of neural structures responsible for incentive salience, associative learning, and positively-valenced emotions, particularly ones involving pleasure as a core component. Reward is the attractive and motivational property of a stimulus that induces appetitive behavior, also known as approach behavior, and consummatory behavior. A rewarding stimulus has been described as "any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward". In operant conditioning, rewarding stimuli function as positive reinforcers; however, the converse statement also holds true: positive reinforcers are rewarding.The reward system motivates animals to approach stimuli or engage in behaviour that increases fitness. Survival for most animal species depends upon maximizing contact with beneficial stimuli and minimizing contact with harmful stimuli. Reward cognition serves to increase the likelihood of survival and reproduction by causing associative learning, eliciting approach and consummatory behavior, and triggering positively-valenced emotions. Thus, reward is a mechanism that evolved to help increase the adaptive fitness of animals. In drug addiction, certain substances over-activate the reward circuit, leading to compulsive substance-seeking behavior resulting from synaptic plasticity in the circuit.

SR-142948 is a drug used in scientific research which is a non-peptide antagonist selective for the neurotensin receptors, although not selective between subtypes.
The ventral pallidum (VP) is a structure within the basal ganglia of the brain. It is an output nucleus whose fibres project to thalamic nuclei, such as the ventral anterior nucleus, the ventral lateral nucleus, and the medial dorsal nucleus. The VP is a core component of the reward system which forms part of the limbic loop of the basal ganglia, a pathway involved in the regulation of motivational salience, behavior, and emotions. It is involved in addiction.
Addiction is a state characterized by compulsive engagement in rewarding stimuli, despite adverse consequences. The process of developing an addiction occurs through instrumental learning, which is otherwise known as operant conditioning.

Meaghan Creed is a Canadian neuroscientist and associate professor of anesthesiology at Washington University in St. Louis. Creed has conducted research on understanding and optimizing deep brain stimulation in the basal ganglia for the treatment of neurological and psychiatric disorders. Her work has been recognized at the national and international level by Pfizer, the American Association for the Advancement of Science (AAAS), the Whitehall Foundation, Brain and Behavior Research Foundation and the Rita Allen Foundation.