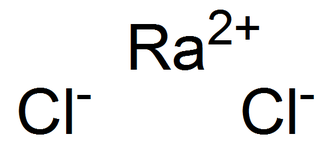
Actinium is a chemical element; it has symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. Actinium gave the name to the actinide series, a set of 15 elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.

Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though longer-lived isotopes exist, such as the 124 years half-life of polonium-209, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.

Radium is a chemical element; it has symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1,600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence.

The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
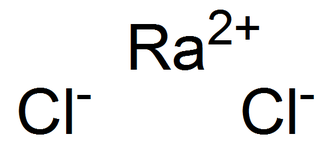
Radium chloride is an inorganic compound with the chemical formula RaCl2. It is a radium salt of hydrogen chloride. It was the first radium compound isolated in a pure state. Marie Curie and André-Louis Debierne used it in their original separation of radium from barium. The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode.

Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs in nature as the mineral barite, which is the main commercial source of barium and materials prepared from it. Its opaque white appearance and its high density are exploited in its main applications.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Barium iodide is an inorganic compound with the formula BaI2. The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids. When heated, hydrated barium iodide converts to the anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
Naturally occurring radioactive materials (NORM) and technologically enhanced naturally occurring radioactive materials (TENORM) consist of materials, usually industrial wastes or by-products enriched with radioactive elements found in the environment, such as uranium, thorium and potassium and any of their decay products, such as radium and radon. Produced water discharges and spills are a good example of entering NORMs into the surrounding environment.

Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.
Friedrich Oskar Giesel was a German organic chemist. During his work in a quinine factory in the late 1890s, he started to work on the at-that-time-new field of radiochemistry and started the production of radium. In the period between 1902 and 1904, he was able to isolate a new element emanium. In a now controversially reviewed process, it was stated that emanium is identical to actinium, which was discovered by André-Louis Debierne in 1899.
The history of radiation therapy or radiotherapy can be traced back to experiments made soon after the discovery of X-rays (1895), when it was shown that exposure to radiation produced cutaneous burns. Influenced by electrotherapy and escharotics—the medical application of caustic substances—doctors began using radiation to treat growths and lesions produced by diseases such as lupus, basal cell carcinoma, and epithelioma. Radiation was generally believed to have bactericidal properties, so when radium was discovered, in addition to treatments similar to those used with x-rays, it was also used as an additive to medical treatments for diseases such as tuberculosis where there were resistant bacilli.
Emile Armet de Lisle (1853–1928) was a French industrialist and chemist who helped develop the French radium industry in the early 20th century. Around the turn of the century, Armet de Lisle began to take notice of a growing market for radium products in France. Seeking to take advantage of this opportunity and leave his own mark on the family business, de Lisle established a new factory, just outside Paris, devoted to the production of radium products in 1904. This was the first radium factory in the world.

Radium sulfate (or radium sulphate) is an inorganic compound with the formula RaSO4 and an average molecular mass of 322.088 g/mol. This white salt is the least soluble of all known sulfate salts. It was formerly used in radiotherapy and smoke detectors, but this has been phased out in favor of less hazardous alternatives.
Radium carbonate is a chemical compound of radium, carbon, and oxygen, having the chemical formula RaCO3. It is the radium salt of carbonic acid. It contains radium cations (Ra2+) and carbonate anions (CO2−3). This salt is a highly radioactive, amorphous, white powder that has potential applications in medicine. It is notable for forming disordered crystals at room temperature and for being approximately 10 times more soluble than the corresponding barium carbonate - witherite. Radium carbonate is one of a few radium compounds which has significantly different properties from corresponding barium compounds. Moreover, radium is the only alkaline-earth metal which forms disordered crystals in its carbonate phase. Even though radium carbonate has very low solubility in water, it is soluble in dilute mineral acids and concentrated ammonium carbonate.

Vitaly Grigorievich Khlopin was a Russian and Soviet scientist- radiochemist, professor, academician of the USSR Academy of Sciences (1939), Hero of Socialist Labour (1949), and director of the Radium Institute of the USSR Academy of Sciences (1939-1950). He was one of the founders of Soviet radiochemistry and radium industry, received the first domestic radium preparations (1921), one of the founders of the Radium Institute and leading participants in the atomic project and founder of the school of Soviet radiochemists.
Radium compounds are compounds containing the element radium (Ra). Due to radium's radioactivity, not many compounds have been well characterized. Solid radium compounds are white as radium ions provide no specific coloring, but they gradually turn yellow and then dark over time due to self-radiolysis from radium's alpha decay. Insoluble radium compounds coprecipitate with all barium, most strontium, and most lead compounds.