
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope: 103Rh. Naturally occurring rhodium is usually found as a free metal or as an alloy with similar metals and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals.

Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula [RhCl(PPh3)3], where 'Ph' denotes a phenyl group). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Rhodium(III) oxide (or Rhodium sesquioxide) is the inorganic compound with the formula Rh2O3. It is a gray solid that is insoluble in ordinary solvents.
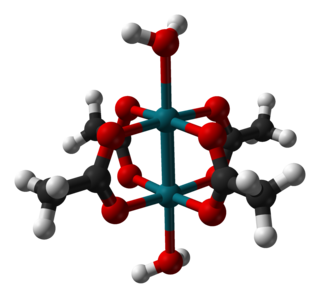
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (CH
3CO−
2). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of alkenes. It is a widely studied example of a transition metal carboxylate complex.
Rhodplumsite is a rare rhodium-lead sulfide mineral, chemical formula Rh3Pb2S2. It was originally discovered within a platinum nugget, in grains up to 40 μm in size. Its name originates from its composition; rhodium and lead (plumbum in Latin). Although this mineral contains large amounts of rhodium, it is not an economically viable ore of rhodium due to its rarity.
Bowieite is a rhodium-iridium-platinum sulfide mineral (Rh,Ir,Pt)2S3, found in platinum-alloy nuggets from Goodnews Bay, Alaska. It was named after the British scientist Stanley Bowie (1917–2008), in recognition of his work on identification of opaque minerals.
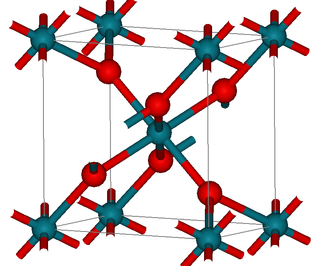
Rhodium(IV) oxide (or rhodium dioxide) is the chemical compound with the formula RhO2.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
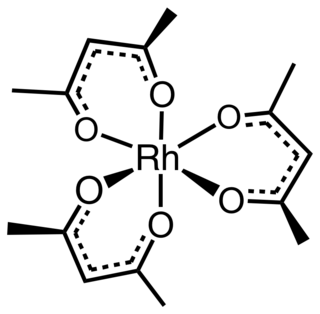
Rhodium acetylacetonate is the coordination complex with the formula Rh(C5H7O2)3, which is sometimes known as Rh(acac)3. The molecule has D3-symmetry. It is a yellow-orange solid that is soluble in organic solvents.

Iridium(III) sulfide is the inorganic compound with the formula Ir2S3. It is an insoluble black solid, prepared by heating a mixture of elemental iridium and sulfur. Crystals can be grown by chemical vapor transport using bromine as the transporting agent. The structure consists of octahedral and tetrahedral Ir and S centers, respectively. No close Ir-Ir contacts are observed. Rh2S3 and Rh2Se3 adopt the same structure.

Rhodium trifluoride is the inorganic compound with the formula RhF3. It is a red-brown, diamagnetic solid.

Dichlorotetrakis(pyridine)rhodium(III) chloride is the chloride salt of the coordination complex with the formula [RhCl2(pyridine)4]+. Various hydrates are known, but all are yellow solids. The tetrahydrate initially crystallizes from water. The tetrahydrate converts to the monohydrate upon vacuum drying at 100 °C.
Rhodium(III) nitrate is a inorganic compound, a salt of rhodium and nitric acid with the formula Rh(NO3)3. This anhydrous complex has been the subject of theoretical analysis but has not been isolated. However, a dihydrate and an aqueous solution are known with similar stoichiometry; they contain various hexacoordinated rhodium(III) aqua and nitrate complexes. A number of other rhodium nitrates have been characterized by X-ray crystallography: Rb4[trans-[Rh(H2O)2(NO3)4][Rh(NO3)6] and Cs2[-[Rh(NO3)5]. Rhodium nitrates are of interest because nuclear wastes, which contain rhodium, are recycled by dissolution in nitric acid.

Rhodium(III) bromide refers to inorganic compounds of the formula RhBr3(H2O)n where n = 0 or approximately three. Both forms are brown solids. The hydrate is soluble in water and lower alcohols. It is used to prepare rhodium bromide complexes. Rhodium bromides are similar to the chlorides, but have attracted little academic or commercial attention.

Rhodium(III) perchlorate refers to the inorganic compound with the formula Rh(H2O)6(ClO4)3. It is a hygroscopic yellow solid. It is the perchlorate salt of the tricationic aquo complex [Rh(H2O)6]3+. The compound is prepared by treating hydrated rhodium(III) chloride and perchloric acid at elevated temperatures:

Rhodium(III) sulfate refers to inorganic compounds of the formula Rh2(SO4)3. It is a red crystalline solid.

Rhodium(III) iodide is an inorganic compound with the formula RhI3. It is a black solid.
Rhodium(III) hydroxide is a chemical compound with the formula Rh(OH)3.
















