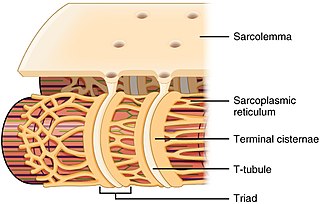
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.

Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited genetic disorder that predisposes those affected to potentially life-threatening abnormal heart rhythms or arrhythmias. The arrhythmias seen in CPVT typically occur during exercise or at times of emotional stress, and classically take the form of bidirectional ventricular tachycardia or ventricular fibrillation. Those affected may be asymptomatic, but they may also experience blackouts or even sudden cardiac death.
A calcium spark is the microscopic release of calcium (Ca2+) from a store known as the sarcoplasmic reticulum (SR), located within muscle cells. This release occurs through an ion channel within the membrane of the SR, known as a ryanodine receptor (RyR), which opens upon activation. This process is important as it helps to maintain Ca2+ concentration within the cell. It also initiates muscle contraction in skeletal and cardiac muscles and muscle relaxation in smooth muscles. Ca2+ sparks are important in physiology as they show how Ca2+ can be used at a subcellular level, to signal both local changes, known as local control, as well as whole cell changes.

Calmodulin 1 is a protein in humans that is encoded by the CALM1 gene.

Protein S100-A1, also known as S100 calcium-binding protein A1 is a protein which in humans is encoded by the S100A1 gene. S100A1 is highly expressed in cardiac and skeletal muscle, and localizes to Z-discs and sarcoplasmic reticulum. S100A1 has shown promise as an effective candidate for gene therapy to treat post-myocardially infarcted cardiac tissue.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.
Peptidyl-prolyl cis-trans isomerase FKBP1B is an enzyme that in humans is encoded by the FKBP1B gene.

Plakophilin-2 is a protein that in humans is encoded by the PKP2 gene. Plakophilin 2 is expressed in skin and cardiac muscle, where it functions to link cadherins to intermediate filaments in the cytoskeleton. In cardiac muscle, plakophilin-2 is found in desmosome structures located within intercalated discs. Mutations in PKP2 have been shown to be causal in arrhythmogenic right ventricular cardiomyopathy.

Triadin, also known as TRDN, is a human gene associated with the release of calcium ions from the sarcoplasmic reticulum triggering muscular contraction through calcium-induced calcium release. Triadin is a multiprotein family, arising from different processing of the TRDN gene on chromosome 6. It is a transmembrane protein on the sarcoplasmic reticulum due to a well defined hydrophobic section and it forms a quaternary complex with the cardiac ryanodine receptor (RYR2), calsequestrin (CASQ2) and junctin proteins. The luminal (inner compartment of the sarcoplasmic reticulum) section of Triadin has areas of highly charged amino acid residues that act as luminal Ca2+ receptors. Triadin is also able to sense luminal Ca2+ concentrations by mediating interactions between RYR2 and CASQ2. Triadin has several different forms; Trisk 95 and Trisk 51, which are expressed in skeletal muscle, and Trisk 32 (CT1), which is mainly expressed in cardiac muscle.

A-kinase anchor protein 6 is an enzyme that in humans is encoded by the AKAP6 gene.

Ryanodine receptor 1 (RYR-1) also known as skeletal muscle calcium release channel or skeletal muscle-type ryanodine receptor is one of a class of ryanodine receptors and a protein found primarily in skeletal muscle. In humans, it is encoded by the RYR1 gene.

Atrial Light Chain-2 (ALC-2) also known as Myosin regulatory light chain 2, atrial isoform (MLC2a) is a protein that in humans is encoded by the MYL7 gene. ALC-2 expression is restricted to cardiac muscle atria in healthy individuals, where it functions to modulate cardiac development and contractility. In human diseases, including hypertrophic cardiomyopathy, dilated cardiomyopathy, ischemic cardiomyopathy and others, ALC-2 expression is altered.

Ankyrin-2, also known as Ankyrin-B, and Brain ankyrin, is a protein which in humans is encoded by the ANK2 gene. Ankyrin-2 is ubiquitously expressed, but shows high expression in cardiac muscle. Ankyrin-2 plays an essential role in the localization and membrane stabilization of ion transporters and ion channels in cardiomyocytes, as well as in costamere structures. Mutations in ANK2 cause a dominantly-inherited, cardiac arrhythmia syndrome known as long QT syndrome 4 as well as sick sinus syndrome; mutations have also been associated to a lesser degree with hypertrophic cardiomyopathy. Alterations in ankyrin-2 expression levels are observed in human heart failure.

Ryanodine receptor 3 is one of a class of ryanodine receptors and a protein that in humans is encoded by the RYR3 gene. The protein encoded by this gene is both a calcium channel and a receptor for the plant alkaloid ryanodine. RYR3 and RYR1 control the resting calcium ion concentration in skeletal muscle.
JTV-519 (K201) is a 1,4-benzothiazepine derivative that interacts with many cellular targets. It has many structural similarities to diltiazem, a Ca2+ channel blocker used for treatment of hypertension, angina pectoris and some types of arrhythmias. JTV-519 acts in the sarcoplasmic reticulum (SR) of cardiac myocytes by binding to and stabilizing the ryanodine receptor (RyR2) in its closed state. It can be used in the treatment of cardiac arrhythmias, heart failure, catecholaminergic polymorphic ventricular tachycardia (CPVT) and store overload-induced Ca2+ release (SOICR). Currently, this drug has only been tested on animals and its side effects are still unknown. As research continues, some studies have also found a dose-dependent response; where there is no improvement seen in failing hearts at 0.3 μM and a decline in response at 1 μM.
CXL 1020 is an experimental drug that is being investigated as a treatment for acute decompensated heart failure. CXL 1020 functions as a nitroxyl donor; nitroxyl is the reduced, protonated version of nitric oxide. Nitroxyl is capable of enhancing left ventricular contractility without increasing heart rate by modifying normal Ca2+ cycling through the sarcoplasmic reticulum as well as increasing the sensitivity of cardiac myofilaments to Ca2+.
The ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channel (RIR-CaC) family includes Ryanodine receptors and Inositol trisphosphate receptors. Members of this family are large proteins, some exceeding 5000 amino acyl residues in length. This family belongs to the Voltage-gated ion channel (VIC) superfamily. Ry receptors occur primarily in muscle cell sarcoplasmic reticular (SR) membranes, and IP3 receptors occur primarily in brain cell endoplasmic reticular (ER) membranes where they effect release of Ca2+ into the cytoplasm upon activation (opening) of the channel. They are redox sensors, possibly providing a partial explanation for how they control cytoplasmic Ca2+. Ry receptors have been identified in heart mitochondria where they provide the main pathway for Ca2+ entry. Sun et al. (2011) have demonstrated oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel (RyR1;TC# 1.A.3.1.2) by NADPH oxidase 4.



















