A premature ventricular contraction (PVC) is a common event where the heartbeat is initiated by Purkinje fibers in the ventricles rather than by the sinoatrial node. PVCs may cause no symptoms or may be perceived as a "skipped beat" or felt as palpitations in the chest. PVCs do not usually pose any danger.

Long QT syndrome (LQTS) is a condition affecting repolarization (relaxing) of the heart after a heartbeat, giving rise to an abnormally lengthy QT interval. It results in an increased risk of an irregular heartbeat which can result in fainting, drowning, seizures, or sudden death. These episodes can be triggered by exercise or stress. Some rare forms of LQTS are associated with other symptoms and signs including deafness and periods of muscle weakness.
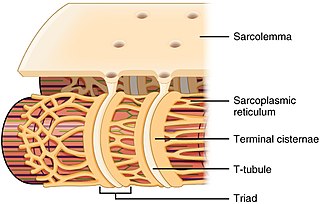
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.
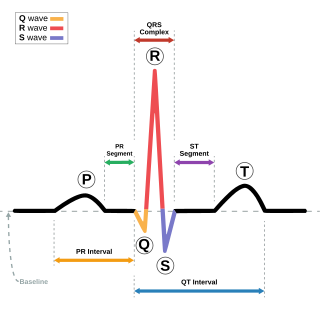
Short QT syndrome (SQT) is a very rare genetic disease of the electrical system of the heart, and is associated with an increased risk of abnormal heart rhythms and sudden cardiac death. The syndrome gets its name from a characteristic feature seen on an electrocardiogram (ECG) – a shortening of the QT interval. It is caused by mutations in genes encoding ion channels that shorten the cardiac action potential, and appears to be inherited in an autosomal dominant pattern. The condition is diagnosed using a 12-lead ECG. Short QT syndrome can be treated using an implantable cardioverter-defibrillator or medications including quinidine. Short QT syndrome was first described in 2000, and the first genetic mutation associated with the condition was identified in 2004.

Ventricular tachycardia is a cardiovascular disorder in which fast heart rate occurs in the ventricles of the heart. Although a few seconds of VT may not result in permanent problems, longer periods are dangerous; and multiple episodes over a short period of time are referred to as an electrical storm. Short periods may occur without symptoms, or present with lightheadedness, palpitations, or chest pain. Ventricular tachycardia may result in ventricular fibrillation (VF) and turn into cardiac arrest. This conversion of the VT into VF is called the degeneration of the VT. It is found initially in about 7% of people in cardiac arrest.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or given by injection into a vein.

Andersen–Tawil syndrome, also called Andersen syndrome and long QT syndrome 7, is a rare genetic disorder affecting several parts of the body. The three predominant features of Andersen–Tawil syndrome include disturbances of the electrical function of the heart characterised by an abnormality seen on an electrocardiogram and a tendency to abnormal heart rhythms, physical characteristics including low-set ears and a small lower jaw, and intermittent periods of muscle weakness known as hypokalaemic periodic paralysis.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.
Calsequestrin is a calcium-binding protein that acts as a calcium buffer within the sarcoplasmic reticulum. The protein helps hold calcium in the cisterna of the sarcoplasmic reticulum after a muscle contraction, even though the concentration of calcium in the sarcoplasmic reticulum is much higher than in the cytosol. It also helps the sarcoplasmic reticulum store an extraordinarily high amount of calcium ions. Each molecule of calsequestrin can bind 18 to 50 Ca2+ ions. Sequence analysis has suggested that calcium is not bound in distinct pockets via EF-hand motifs, but rather via presentation of a charged protein surface. Two forms of calsequestrin have been identified. The cardiac form Calsequestrin-2 (CASQ2) is present in cardiac and slow skeletal muscle and the fast skeletal form Calsequestrin-1(CASQ1) is found in fast skeletal muscle. The release of calsequestrin-bound calcium (through a calcium release channel) triggers muscle contraction. The active protein is not highly structured, more than 50% of it adopting a random coil conformation. When calcium binds there is a structural change whereby the alpha-helical content of the protein increases from 3 to 11%. Both forms of calsequestrin are phosphorylated by casein kinase 2, but the cardiac form is phosphorylated more rapidly and to a higher degree. Calsequestrin is also secreted in the gut where it deprives bacteria of calcium ions..
Afterdepolarizations are abnormal depolarizations of cardiac myocytes that interrupt phase 2, phase 3, or phase 4 of the cardiac action potential in the electrical conduction system of the heart. Afterdepolarizations may lead to cardiac arrhythmias. Afterdepolarization is commonly a consequence of myocardial infarction, cardiac hypertrophy, or heart failure. It may also result from congenital mutations associated with calcium channels and sequestration.

Calmodulin 1 is a protein in humans that is encoded by the CALM1 gene.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.
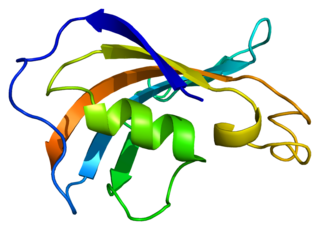
Peptidyl-prolyl cis-trans isomerase FKBP1B is an enzyme that in humans is encoded by the FKBP1B gene.

Triadin, also known as TRDN, is a human gene associated with the release of calcium ions from the sarcoplasmic reticulum triggering muscular contraction through calcium-induced calcium release. Triadin is a multiprotein family, arising from different processing of the TRDN gene on chromosome 6. It is a transmembrane protein on the sarcoplasmic reticulum due to a well defined hydrophobic section and it forms a quaternary complex with the cardiac ryanodine receptor (RYR2), calsequestrin (CASQ2) and junctin proteins. The luminal (inner compartment of the sarcoplasmic reticulum) section of Triadin has areas of highly charged amino acid residues that act as luminal Ca2+ receptors. Triadin is also able to sense luminal Ca2+ concentrations by mediating interactions between RYR2 and CASQ2. Triadin has several different forms; Trisk 95 and Trisk 51, which are expressed in skeletal muscle, and Trisk 32 (CT1), which is mainly expressed in cardiac muscle.

Ankyrin-2, also known as Ankyrin-B, and Brain ankyrin, is a protein which in humans is encoded by the ANK2 gene. Ankyrin-2 is ubiquitously expressed, but shows high expression in cardiac muscle. Ankyrin-2 plays an essential role in the localization and membrane stabilization of ion transporters and ion channels in cardiomyocytes, as well as in costamere structures. Mutations in ANK2 cause a dominantly-inherited, cardiac arrhythmia syndrome known as long QT syndrome 4 as well as sick sinus syndrome; mutations have also been associated to a lesser degree with hypertrophic cardiomyopathy. Alterations in ankyrin-2 expression levels are observed in human heart failure.
JTV-519 (K201) is a 1,4-benzothiazepine derivative that interacts with many cellular targets. It has many structural similarities to diltiazem, a Ca2+ channel blocker used for treatment of hypertension, angina pectoris and some types of arrhythmias. JTV-519 acts in the sarcoplasmic reticulum (SR) of cardiac myocytes by binding to and stabilizing the ryanodine receptor (RyR2) in its closed state. It can be used in the treatment of cardiac arrhythmias, heart failure, catecholaminergic polymorphic ventricular tachycardia (CPVT) and store overload-induced Ca2+ release (SOICR). Currently, this drug has only been tested on animals and its side effects are still unknown. As research continues, some studies have also found a dose-dependent response; where there is no improvement seen in failing hearts at 0.3 μM and a decline in response at 1 μM.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.
Calcium buffering describes the processes which help stabilise the concentration of free calcium ions within cells, in a similar manner to how pH buffers maintain a stable concentration of hydrogen ions. The majority of calcium ions within the cell are bound to intracellular proteins, leaving a minority freely dissociated. When calcium is added to or removed from the cytoplasm by transport across the cell membrane or sarcoplasmic reticulum, calcium buffers minimise the effect on changes in cytoplasmic free calcium concentration by binding calcium to or releasing calcium from intracellular proteins. As a result, 99% of the calcium added to the cytosol of a cardiomyocyte during each cardiac cycle becomes bound to calcium buffers, creating a relatively small change in free calcium.