Myotonia is a symptom of a small handful of certain neuromuscular disorders characterized by delayed relaxation of the skeletal muscles after voluntary contraction or electrical stimulation, and the muscle shows an abnormal EMG.
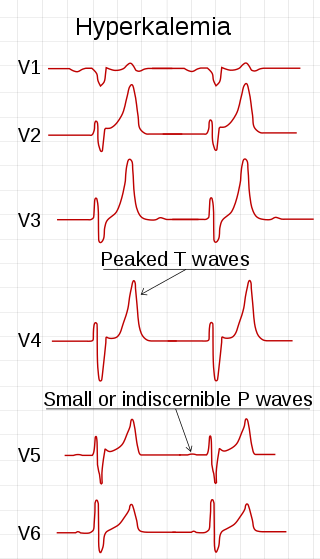
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.
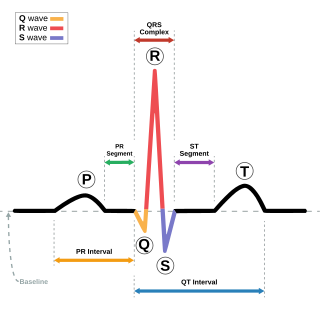
Romano–Ward syndrome is the most common form of congenital Long QT syndrome (LQTS), a genetic heart condition that affects the electrical properties of heart muscle cells. Those affected are at risk of abnormal heart rhythms which can lead to fainting, seizures, or sudden death. Romano–Ward syndrome can be distinguished clinically from other forms of inherited LQTS as it affects only the electrical properties of the heart, while other forms of LQTS can also affect other parts of the body.

Andersen–Tawil syndrome, also called Andersen syndrome and long QT syndrome 7, is a rare genetic disorder affecting several parts of the body. The three predominant features of Andersen–Tawil syndrome include disturbances of the electrical function of the heart characterised by an abnormality seen on an electrocardiogram and a tendency to abnormal heart rhythms, physical characteristics including low-set ears and a small lower jaw, and intermittent periods of muscle weakness known as hypokalaemic periodic paralysis.

Channelopathies are a group of diseases caused by the dysfunction of ion channel subunits or their interacting proteins. These diseases can be inherited or acquired by other disorders, drugs, or toxins. Mutations in genes encoding ion channels, which impair channel function, are the most common cause of channelopathies. There are more than 400 genes that encode ion channels, found in all human cell types and are involved in almost all physiological processes. Each type of channel is a multimeric complex of subunits encoded by a number of genes. Depending where the mutation occurs it may affect the gating, conductance, ion selectivity, or signal transduction of the channel.
Myotonia congenita is a congenital neuromuscular channelopathy that affects skeletal muscles. It is a genetic disorder. The hallmark of the disease is the failure of initiated contraction to terminate, often referred to as delayed relaxation of the muscles (myotonia) and rigidity. Symptoms include delayed relaxation of the muscles after voluntary contraction (myotonia), and may also include stiffness, hypertrophy (enlargement), transient weakness in some forms of the disorder, severe masseter spasm, and cramping. The condition is sometimes referred to as fainting goat syndrome, as it is responsible for the eponymous 'fainting' seen in fainting goats when presented with a sudden stimulus. Of note, myotonia congenita has no association with malignant hyperthermia (MH).
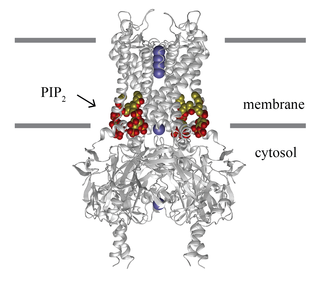
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.
Periodic paralysis is a group of rare genetic diseases that lead to weakness or paralysis from common triggers such as cold, heat, high carbohydrate meals, not eating, stress or excitement and physical activity of any kind. The underlying mechanism of these diseases are malfunctions in the ion channels in skeletal muscle cell membranes that allow electrically charged ions to leak in or out of the muscle cell, causing the cell to depolarize and become unable to move.

Hypokalemic periodic paralysis (hypoKPP), also known as familial hypokalemic periodic paralysis (FHPP), is a rare, autosomal dominant channelopathy characterized by muscle weakness or paralysis when there is a fall in potassium levels in the blood. In individuals with this mutation, attacks sometimes begin in adolescence and most commonly occur with individual triggers such as rest after strenuous exercise, high carbohydrate meals, meals with high sodium content, sudden changes in temperature, and even excitement, noise, flashing lights, cold temperatures and stress. Weakness may be mild and limited to certain muscle groups, or more severe full-body paralysis. During an attack, reflexes may be decreased or absent. Attacks may last for a few hours or persist for several days. Recovery is usually sudden when it occurs, due to release of potassium from swollen muscles as they recover. Some patients may fall into an abortive attack or develop chronic muscle weakness later in life.

Paramyotonia congenita (PC) is a rare congenital autosomal dominant neuromuscular disorder characterized by "paradoxical" myotonia. This type of myotonia has been termed paradoxical because it becomes worse with exercise whereas classical myotonia, as seen in myotonia congenita, is alleviated by exercise. PC is also distinguished as it can be induced by cold temperatures. Although more typical of the periodic paralytic disorders, patients with PC may also have potassium-provoked paralysis. PC typically presents within the first decade of life and has 100% penetrance. Patients with this disorder commonly present with myotonia in the face or upper extremities. The lower extremities are generally less affected. While some other related disorders result in muscle atrophy, this is not normally the case with PC. This disease can also present as hyperkalemic periodic paralysis and there is debate as to whether the two disorders are actually distinct.

Sodium channel protein type 4 subunit alpha is a protein that in humans is encoded by the SCN4A gene.
Sodium channel protein type 5 subunit alpha, also known as NaV1.5 is an integral membrane protein and tetrodotoxin-resistant voltage-gated sodium channel subunit. NaV1.5 is found primarily in cardiac muscle, where it mediates the fast influx of Na+-ions (INa) across the cell membrane, resulting in the fast depolarization phase of the cardiac action potential. As such, it plays a major role in impulse propagation through the heart. A vast number of cardiac diseases is associated with mutations in NaV1.5 (see paragraph genetics). SCN5A is the gene that encodes the cardiac sodium channel NaV1.5.

Nav1.7 is a sodium ion channel that in humans is encoded by the SCN9A gene. It is usually expressed at high levels in two types of neurons: the nociceptive (pain) neurons at dorsal root ganglion (DRG) and trigeminal ganglion and sympathetic ganglion neurons, which are part of the autonomic (involuntary) nervous system.

Potassium-aggravated myotonia is a rare genetic disorder that affects skeletal muscle. Beginning in childhood or adolescence, people with this condition experience bouts of sustained muscle tensing (myotonia) that prevent muscles from relaxing normally. Myotonia causes muscle stiffness, often painful, that worsens after exercise and may be aggravated by eating potassium-rich foods such as bananas and potatoes. Stiffness occurs in skeletal muscles throughout the body. Potassium-aggravated myotonia ranges in severity from mild episodes of muscle stiffness to severe, disabling disease with frequent attacks. Potassium-aggravated myotonia may, in some cases, also cause paradoxical myotonia, in which myotonia becomes more severe at the time of movement instead of after movement has ceased. Unlike some other forms of myotonia, potassium-aggravated myotonia is not associated with episodes of muscle weakness.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.

Thyrotoxic periodic paralysis (TPP) is a rare condition featuring attacks of muscle weakness in the presence of hyperthyroidism. Hypokalemia is usually present during attacks. The condition may be life-threatening if weakness of the breathing muscles leads to respiratory failure, or if the low potassium levels lead to abnormal heart rhythms. If untreated, it is typically recurrent in nature.
The Kir2.6 also known as inward rectifier potassium channel 18 is a protein that in humans is encoded by the KCNJ18 gene. Kir2.6 is an inward-rectifier potassium ion channel.
Louis Ptáček is an American neurologist and professor who contributed greatly to the field of genetics and neuroscience. He was also an HHMI investigator from 1997 to 2018. His chief areas of research include the understanding of inherited Mendelian disorders and circadian rhythm genes. Currently, Ptáček is a neurology professor and a director of the Division of Neurogenetics in University of California, San Francisco, School of Medicine. His current investigations primarily focus on extensive clinical studies in families with hereditary disorders, which include identifying and characterizing the genes responsible for neurological variations.
Hyperkalemic periodic paralysis is a genetic disorder that occurs in horses. It is also known as Impressive syndrome, after an index case in a horse named Impressive. It is an inherited autosomal dominant disorder that affects sodium channels in muscle cells and the ability to regulate potassium levels in the blood. It is characterized by muscle hyperexcitability or weakness which, exacerbated by potassium, heat or cold, can lead to uncontrolled shaking followed by paralysis.









