A testicle or testis is the male reproductive gland or gonad in all bilaterians, including humans. It is homologous to the female ovary. The functions of the testes are to produce both sperm and androgens, primarily testosterone. Testosterone release is controlled by the anterior pituitary luteinizing hormone, whereas sperm production is controlled both by the anterior pituitary follicle-stimulating hormone and gonadal testosterone.

Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testis. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules. These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.

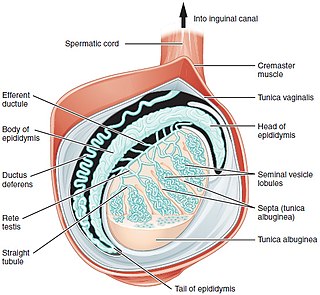
Seminiferous tubules are located within the testes, and are the specific location of meiosis, and the subsequent creation of male gametes, namely spermatozoa.

Sertoli cells are a type of sustentacular "nurse" cell found in human testes which contribute to the process of spermatogenesis as a structural component of the seminiferous tubules. They are activated by follicle-stimulating hormone (FSH) secreted by the adenohypophysis and express FSH receptor on their membranes.

Spermatocytes are a type of male gametocyte in animals. They derive from immature germ cells called spermatogonia. They are found in the testis, in a structure known as the seminiferous tubules. There are two types of spermatocytes, primary and secondary spermatocytes. Primary and secondary spermatocytes are formed through the process of spermatocytogenesis.

The male reproductive system consists of a number of sex organs that play a role in the process of human reproduction. These organs are located on the outside of the body and within the pelvis.

Azoospermia is the medical condition of a man whose semen contains no sperm. It is associated with male infertility, but many forms are amenable to medical treatment. In humans, azoospermia affects about 1% of the male population and may be seen in up to 20% of male infertility situations in Canada.
Terms oligospermia, oligozoospermia, and low sperm count refer to semen with a low concentration of sperm and is a common finding in male infertility. Often semen with a decreased sperm concentration may also show significant abnormalities in sperm morphology and motility. There has been interest in replacing the descriptive terms used in semen analysis with more quantitative information.
Y chromosome microdeletion(YCM) is a family of genetic disorders caused by missing genes in the Y chromosome. Many men with YCM exhibit no symptoms and lead normal lives. It is present in a significant number of men with reduced fertility. Reduced sperm production varies from oligozoospermia, significant lack of sperm, or azoospermia, complete lack of sperm.
Male infertility refers to a sexually mature male's inability to impregnate a fertile female. In humans it accounts for 40–50% of infertility. It affects approximately 7% of all men. Male infertility is commonly due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity. More recently, advance sperm analyses that examine intracellular sperm components are being developed.
Spermatogenesis arrest is known as the interruption of germinal cells of specific cellular type, which elicits an altered spermatozoa formation. Spermatogenic arrest is usually due to genetic factors resulting in irreversible azoospermia. However some cases may be consecutive to hormonal, thermic, or toxic factors and may be reversible either spontaneously or after a specific treatment. Spermatogenic arrest results in either oligospermia or azoospermia in men. It is quite a difficult condition to proactively diagnose as it tends to affect those who have normal testicular volumes; a diagnosis can be made however through a testicular biopsy.

Testicular sperm extraction (TESE) is a surgical procedure in which a small portion of tissue is removed from the testicle and any viable sperm cells from that tissue are extracted for use in further procedures, most commonly intracytoplasmic sperm injection (ICSI) as part of in vitro fertilisation (IVF). TESE is often recommended to patients who cannot produce sperm by ejaculation due to azoospermia.
Azoospermia factor (AZF) is one of several proteins or their genes, which are coded from the AZF region on the human male Y chromosome. Deletions in this region are associated with inability to produce sperm. Subregions within the AZF region are AZFa, AZFb and AZFc. AZF microdeletions are one of the major causes of male infertility for azoospermia and severe oligozoospermia males. AZF is the term used by the HUGO Gene Nomenclature Committee.

Adjudin (AF-2364) is a drug which is under development as a potential non-hormonal male contraceptive drug, which acts by blocking the production of sperm in the testes, but without affecting testosterone production. It is an analogue of the chemotherapy drug lonidamine, an indazole-carboxylic acid, and further studies continue to be conducted into this family of drugs as possible contraceptives.

Ubiquitin specific peptidase 9, Y-linked , also known as USP9Y, is an enzyme which in humans is encoded by the USP9Y gene. It is required for sperm production. This enzyme is a member of the peptidase C19 family and is similar to ubiquitin-specific proteases, which cleave the ubiquitin moiety from ubiquitin-fused precursors and ubiquitinylated proteins.
Testicular Immunology is the study of the immune system within the testis. It includes an investigation of the effects of infection, inflammation and immune factors on testicular function. Two unique characteristics of testicular immunology are evident: (1) the testis is described as an immunologically privileged site, where suppression of immune responses occurs; and, (2) some factors which normally lead to inflammation are present at high levels in the testis, where they regulate the development of sperm instead of promoting inflammation.
FNA mapping is an application of fine-needle aspiration (FNA) to the testis for the diagnosis of male infertility. FNA cytology has been used to examine pathological human tissue from various organs for over 100 years. As an alternative to open testicular biopsy for the last 40 years, FNA mapping has helped to characterize states of human male infertility due to defective spermatogenesis. Although recognized as a reliable, and informative technique, testis FNA has not been widely used in U.S. to evaluate male infertility. Recently, however, testicular FNA has gained popularity as both a diagnostic and therapeutic tool for the management of clinical male infertility for several reasons:
- The testis is an ideal organ for evaluation by FNA because of its uniform cellularity and easy accessibility.
- The trend toward minimally invasive procedures and cost-containment views FNA favorably compared to surgical testis biopsy.
- The realization that the specific histologic abnormality observed on testis biopsy has no definite correlation to either the etiology of infertility or to the ability to find sperm for assisted reproduction.
- Assisted reproduction has undergone dramatic advances such that testis sperm are routinely used for biological pregnancies, thus fueling the development of novel FNA techniques to both locate and procure sperm.
Follicle-stimulating hormone (FSH) insensitivity, or ovarian insensitivity to FSH in females, also referable to as ovarian follicle hypoplasia or granulosa cell hypoplasia in females, is a rare autosomal recessive genetic and endocrine syndrome affecting both females and males, with the former presenting with much greater severity of symptomatology. It is characterized by a resistance or complete insensitivity to the effects of follicle-stimulating hormone (FSH), a gonadotropin which is normally responsible for the stimulation of estrogen production by the ovaries in females and maintenance of fertility in both sexes. The condition manifests itself as hypergonadotropic hypogonadism, reduced or absent puberty, amenorrhea, and infertility in females, whereas males present merely with varying degrees of infertility and associated symptoms.

A spermatogonial stem cell (SSC), also known as a type A spermatogonium, is a spermatogonium that does not differentiate into a spermatocyte, a precursor of sperm cells. Instead, they continue dividing into other spermatogonia or remain dormant to maintain a reserve of spermatogonia. Type B spermatogonia, on the other hand, differentiate into spermatocytes, which in turn undergo meiosis to eventually form mature sperm cells.
In vitro spermatogenesis is the process of creating male gametes (spermatozoa) outside of the body in a culture system. The process could be useful for fertility preservation, infertility treatment and may further develop the understanding of spermatogenesis at the cellular and molecular level.










