
Escherichia coli ( ESH-ə-RIK-ee-ə KOH-ly) is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms. Most E. coli strains are harmless, but some serotypes such as EPEC, and ETEC are pathogenic and can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains are part of the normal microbiota of the gut and are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of E. coli benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between E. coli and humans are a type of mutualistic biological relationship — where both the humans and the E. coli are benefitting each other. E. coli is expelled into the environment within fecal matter. The bacterium grows massively in fresh fecal matter under aerobic conditions for three days, but its numbers decline slowly afterwards.

Escherichia coli O157:H7 is a serotype of the bacterial species Escherichia coli and is one of the Shiga-like toxin–producing types of E. coli. It is a cause of disease, typically foodborne illness, through consumption of contaminated and raw food, including raw milk and undercooked ground beef. Infection with this type of pathogenic bacteria may lead to hemorrhagic diarrhea, and to kidney failure; these have been reported to cause the deaths of children younger than five years of age, of elderly patients, and of patients whose immune systems are otherwise compromised.

Shiga toxins are a family of related toxins with two major groups, Stx1 and Stx2, expressed by genes considered to be part of the genome of lambdoid prophages. The toxins are named after Kiyoshi Shiga, who first described the bacterial origin of dysentery caused by Shigella dysenteriae. Shiga-like toxin (SLT) is a historical term for similar or identical toxins produced by Escherichia coli. The most common sources for Shiga toxin are the bacteria S. dysenteriae and some serotypes of Escherichia coli (STEC), which includes serotypes O157:H7, and O104:H4.

Hemolytic–uremic syndrome (HUS) is a group of blood disorders characterized by low red blood cells, acute kidney injury, and low platelets. Initial symptoms typically include bloody diarrhea, fever, vomiting, and weakness. Kidney problems and low platelets then occur as the diarrhea progresses. Children are more commonly affected, but most children recover without permanent damage to their health, although some children may have serious and sometimes life-threatening complications. Adults, especially the elderly, may present a more complicated presentation. Complications may include neurological problems and heart failure.

Coliform bacteria are defined as either motile or non-motile Gram-negative non-spore forming bacilli that possess β-galactosidase to produce acids and gases under their optimal growth temperature of 35–37 °C. They can be aerobes or facultative aerobes, and are a commonly used indicator of low sanitary quality of foods, milk, and water. Coliforms can be found in the aquatic environment, in soil and on vegetation; they are universally present in large numbers in the feces of warm-blooded animals as they are known to inhabit the gastrointestinal system. While coliform bacteria are not normally causes of serious illness, they are easy to culture, and their presence is used to infer that other pathogenic organisms of fecal origin may be present in a sample, or that said sample is not safe to consume. Such pathogens include disease-causing bacteria, viruses, or protozoa and many multicellular parasites.
The AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria known to cause human diseases such as cholera, dysentery, and hemolytic–uremic syndrome. One component is known as the A subunit, and the remaining five components are B subunits. All of these toxins share a similar structure and mechanism for entering targeted host cells. The B subunit is responsible for binding to receptors to open up a pathway for the A subunit to enter the cell. The A subunit is then able to use its catalytic machinery to take over the host cell's regular functions.

Intimin is a virulence factor (adhesin) of EPEC and EHEC E. coli strains. It is an attaching and effacing (A/E) protein, which with other virulence factors is necessary and responsible for enteropathogenic and enterohaemorrhagic diarrhoea.
Escherichia coli O121 is a pathogenic serotype of Escherichia coli, associated with Shiga toxin, intestinal bleeding, and hemolytic-uremic syndrome (HUS). HUS, if left untreated, can lead to kidney failure.
Escherichia coli O104:H21 is a rare serotype of Escherichia coli, a species of bacteria that lives in the lower intestines of mammals. Although there are many serotypes of E. coli, when in animals, there are benefits or do not cause disease. Some serotypes of E. coli have been recognized as pathogenic to humans, e.g. E. coli O157:H7, E. coli O121 and E. coli O104:H21.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxins. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.
Enteroinvasive Escherichia coli (EIEC) is a type of pathogenic bacteria whose infection causes a syndrome that is identical to shigellosis, with profuse diarrhea and high fever. EIEC are highly invasive, and they use adhesin proteins to bind to and enter intestinal cells. They produce no toxins, but severely damage the intestinal wall through mechanical cell destruction.
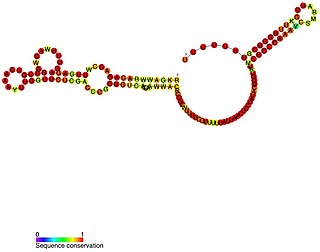
VrrA is a non-coding RNA that is conserved across all Vibrio species of bacteria and acts as a repressor for the synthesis of the outer membrane protein OmpA. This non-coding RNA was initially identified from Tn5 transposon mutant libraries of Vibrio cholerae and its location within the bacterial genome was mapped to the intergenic region between genes VC1741 and VC1743 by RACE analysis.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.

A novel strain of Escherichia coli O104:H4 bacteria caused a serious outbreak of foodborne illness focused in northern Germany in May through June 2011. The illness was characterized by bloody diarrhea, with a high frequency of serious complications, including hemolytic–uremic syndrome (HUS), a condition that requires urgent treatment. The outbreak was originally thought to have been caused by an enterohemorrhagic (EHEC) strain of E. coli, but it was later shown to have been caused by an enteroaggregative E. coli (EAEC) strain that had acquired the genes to produce Shiga toxins, present in organic fenugreek sprouts.
Escherichia coli O104:H4 is an enteroaggregative Escherichia coli strain of the bacterium Escherichia coli, and the cause of the 2011 Escherichia coli O104:H4 outbreak. The "O" in the serological classification identifies the cell wall lipopolysaccharide antigen, and the "H" identifies the flagella antigen.
Antimotility agents are drugs used to alleviate the symptoms of diarrhea. These include loperamide (Imodium), diphenoxylate with atropine (Lomotil), and opiates such as paregoric, tincture of opium, codeine, and morphine. In diarrhea caused by invasive pathogens such as Salmonella, Shigella, and Campylobacter, the use of such agents has generally been strongly discouraged, though evidence is lacking that they are harmful when administered in combination with antibiotics in Clostridium difficile cases. Use of antimotility agents in children and the elderly has also been discouraged in treatment of EHEC due to an increased rate of hemolytic uremic syndrome.

Escherichia coli is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but pathogenic varieties cause serious food poisoning, septic shock, meningitis, or urinary tract infections in humans. Unlike normal flora E. coli, the pathogenic varieties produce toxins and other virulence factors that enable them to reside in parts of the body normally not inhabited by E. coli, and to damage host cells. These pathogenic traits are encoded by virulence genes carried only by the pathogens.
Enteroaggregative Escherichia coli are a pathotype of Escherichia coli which cause acute and chronic diarrhea in both the developed and developing world. They may also cause urinary tract infections. EAEC are defined by their "stacked-brick" pattern of adhesion to the human laryngeal epithelial cell line HEp-2. The pathogenesis of EAEC involves the aggregation of and adherence of the bacteria to the intestinal mucosa, where they elaborate enterotoxins and cytotoxins that damage host cells and induce inflammation that results in diarrhea.
Proteobiotics are natural metabolites which are produced by fermentation process of specific probiotic strains. These small oligopeptides were originally discovered in and isolated from culture media used to grow probiotic bacteria and may account for some of the health benefits of probiotics.

![Inactivation of LEE genes ( | [fucose] ) INACTIVATION OF LEE GENES.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e9/INACTIVATION_OF_LEE_GENES.jpg/220px-INACTIVATION_OF_LEE_GENES.jpg)
![Activation of LEE genes ( | [fucose] ) ACTIVATION OF LEE GENES.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8b/ACTIVATION_OF_LEE_GENES.jpg/220px-ACTIVATION_OF_LEE_GENES.jpg)






