
In organic chemistry, radical anion is a subset of charged free radical species that carry a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Ferric acetate is the acetate salt of the coordination complex [Fe3O(OAc)6(H2O)3]+ (OAc− is CH3CO2−). Commonly the salt is known as "basic iron acetate". The formation of the red-brown complex was once used as a test for ferric ions.

Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The ReH2−
9 anion is a rare example of a coordination complex bearing only hydride ligands.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.

Roussin's Red Salt is the inorganic compound with the formula K2[Fe2S2(NO)4]. This metal nitrosyl was first described by Zacharie Roussin in 1858, making it one of the first synthetic iron-sulfur clusters.
Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration, corrosion, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.
In inorganic chemistry, olation is the process by which metal ions form polymeric oxides in aqueous solution. The phenomenon is important for understanding the relationship between metal aquo complexes and metal oxides, which are represented by many minerals.

In organometallic chemistry, a dicarbollide is an anion of the formula [C2B9H11]2-. Various isomers exist, but most common is 1,2-dicarbollide derived from ortho-carborane. These dianions function as ligands, related to the cyclopentadienyl anion. Substituted dicarbollides are also known such as [C2B9H10(pyridine)]− (pyridine bonded to B) and [C2R2B9H9]2- (R groups bonded to carbon).
Metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand, but of course many complexes are known to consist of a mix of aquo and other ligands.
Molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, X = F, Cl, Br, I. The element A may be a main group element, a transition element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.
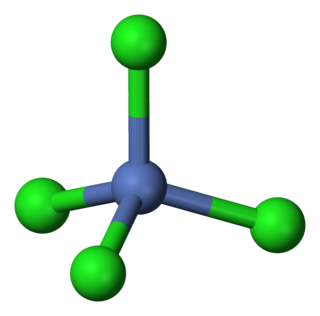
Tetrachloronickelate is the metal complex with the formula [NiCl4]2−. Salts of the complex are available with a variety of cations, but a common one is tetraethylammonium.
Compounds of nickel are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

Tetrabutylammonium is a quaternary ammonium cation with the formula [N(C4H9)4]+. It is used in the research laboratory to prepare lipophilic salts of inorganic anions. Relative to tetraethylammonium derivatives, tetrabutylammonium salts are more lipophilic but crystallize less readily.
The tetrabromonickelate anion contains a doubly-charged nickel atom (Ni2+) surrounded by four bromide ions in a tetrahedral arrangement. The formula is [NiBr4]2−.

Chromium(III) acetate, commonly known as basic chromium acetate, describes a family of salts where the cation has the formula [Cr3O(O2CCH3)6(OH2)3]+. The trichromium cation is encountered with a variety of anions, such as chloride and nitrate. Data in the table above are for the chloride hexahydrate, [Cr3O(O2CCH3)6(OH2)3]Cl(H2O)6.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Sodium trimethylsiloxide is an organosilicon compound with the formula NaOSi(CH3)3. It is the sodium salt of the conjugate base derived from trimethylsilanol. A white solid, its molecular structure consists of a cluster with Na-O-Na linkages on the basis of closely related compounds.

Tetraethylammonium tetrachloroferrate is the chemical compound with the formula (N(C2H5)4)FeCl4. It is the tetraethylammonium salt of the tetrahedral anion [FeCl4]−.

Transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite (NO2−) ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.














