Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and second-highest boiling point of any element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. They are manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All known elements of group 7 are transition metals.

The glycine receptor is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.
Christopher Francis Higgins is a British molecular biologist, geneticist, academic and scientific advisor. He was the Vice-Chancellor of Durham University from 2007 to 2014. He took early retirement on 30 September 2014, following a discussion at Senate on limiting the powers of the Vice Chancellor. He was previously the director of the MRC Clinical Sciences Centre and Head of Division in the Faculty of Medicine at Imperial College London.
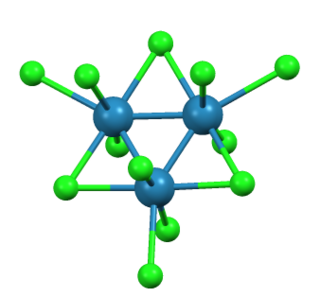
Trirhenium nonachloride is a compound with the formula ReCl3, sometimes also written Re3Cl9. It is a dark red hygroscopic solid that is insoluble in ordinary solvents. The compound is important in the history of inorganic chemistry as an early example of a cluster compound with metal-metal bonds. It is used as a starting material for synthesis of other rhenium complexes.
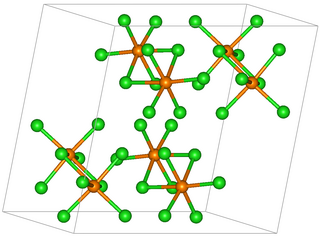
Rhenium pentachloride is an inorganic compound of chlorine and rhenium. The compound has the formula Re2Cl10 but it is usually referred to as rhenium pentachloride. It is a red-brown solid.

The CLCN5 gene encodes the chloride channel Cl-/H+ exchanger ClC-5. ClC-5 is mainly expressed in the kidney, in particular in proximal tubules where it participates to the uptake of albumin and low-molecular-weight proteins, which is one of the principal physiological role of proximal tubular cells. Mutations in the CLCN5 gene cause an X-linked recessive nephropathy named Dent disease characterized by excessive urinary loss of low-molecular-weight proteins and of calcium (hypercalciuria), nephrocalcinosis and nephrolithiasis.

Chloride channel Kb, also known as CLCNKB, is a protein which in humans is encoded by the CLCNKB gene.

Chloride intracellular channel protein 2 is a protein that in humans is encoded by the CLIC2 gene.
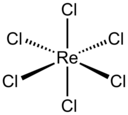
Rhenium chloride may refer to:

PR domain zinc finger protein 9 is a protein that in humans is encoded by the PRDM9 gene. PRDM9 is responsible for positioning recombination hotspots during meiosis by binding a DNA sequence motif encoded in its zinc finger domain. PRDM9 is the only speciation gene found so far in mammals, and is one of the fastest evolving genes in the genome.
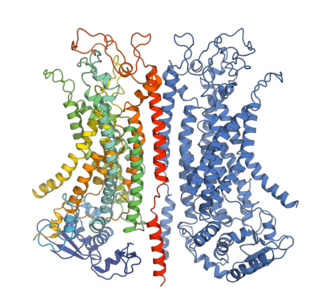
The Calcium-Dependent Chloride Channel (Ca-ClC) proteins (or calcium-activated chloride channels, are heterogeneous groups of ligand-gated ion channels for chloride that have been identified in many epithelial and endothelial cell types as well as in smooth muscle cells. They include proteins from several structurally different families: chloride channel accessory, bestrophin, and calcium-dependent chloride channel anoctamin channels ANO1 is highly expressed in human gastrointestinal interstitial cells of Cajal, which are proteins which serve as intestinal pacemakers for peristalsis. In addition to their role as chloride channels some CLCA proteins function as adhesion molecules and may also have roles as tumour suppressors. These eukaryotic proteins are "required for normal electrolyte and fluid secretion, olfactory perception, and neuronal and smooth muscle excitability" in animals. Members of the Ca-CIC family are generally 600 to 1000 amino acyl residues in length and exhibit 7 to 10 transmembrane segments.

Rhenium(IV) chloride is the inorganic compound with the formula ReCl4. This black solid is of interest as a binary phase but otherwise is of little practical value. A second polymorph of ReCl4 is also known.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +6, +4, and +2 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. Tetrathioperrhenate anion [ReS4]− is possible.