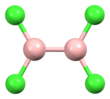
Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.
Cyclopropene is an organic compound with the formula C3H4. It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit.

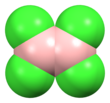
Cerium(III) chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic salt; it rapidly absorbs water on exposure to moist air to form a hydrate, which appears to be of variable composition, though the heptahydrate CeCl3·7H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol and acetone.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.
The Sakurai reaction is the chemical reaction of carbon electrophiles with allyltrimethylsilane catalyzed by strong Lewis acids. The reaction achieves results similar to the addition of an allyl Grignard reagent to the carbonyl.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
Diboron tetrafluoride is the inorganic compound with the formula (BF2)2. A colorless gas, the compound has a halflife of days at room temperature. It is the most stable of the diboron tetrahalides, and does not appreciably decompose under standard conditions.
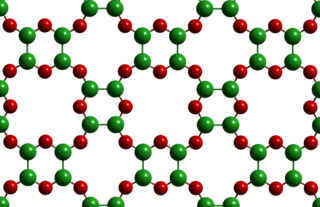
Boron monoxide (BO) is a binary compound of boron and oxygen. It has a molar mass of 26.81 g/mol. The material was first reported in 1940, with a modified synthetic procedure published in 1955, however, the material's structure had remained unknown for nearly a century. A number of allotropes of BO have been theorized ranging from molecular species, to 1D, 2D, and 3D-structured materials, but these were difficult to differentiate using common structural characterization methods. Recent work suggests that the material forms 2D nanosheets composed of O-bridged B4O2 rings, a structure initially postulated in 1961. Due to the lack of precise structural information on the identity of the compound, it has not found widespread use in industry.
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest.
Frederick Nye Tebbe was a chemist known for his work on organometallic chemistry. Tebbe was born in Oakland, California on March 20, 1935. His father, Charles L. Tebbe, worked for the United States Forest Service so Fred’s early education took place in Montana, Oregon, Maryland and Pennsylvania. He married Margaret Manzer in 1960, and they had a son and a daughter. He died of pancreatic cancer at his home in Delaware on September 28, 1995.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.
Diborane(2), also known as diborene, is an inorganic compound with the formula B2H2. The number 2 in diborane(2) indicates the number of hydrogen atoms bonded to the boron complex. There are other forms of diborane with different numbers of hydrogen atoms, including diborane(4) and diborane(6).

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
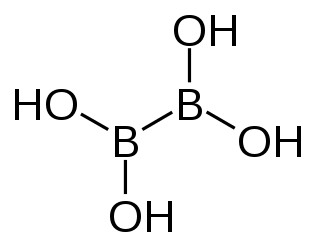
Tetrahydroxydiboron is a chemical reagent which can be used to prepare boronic acids.

Tetravinyltin (also known as tetravinylstannane) is an organotin compound with a chemical formula of C8H12Sn.
Manganese(III) chloride is the hypothetical inorganic compound with the formula MnCl3.

Tetrahalodiboranes are a class of diboron compounds with the formula . These compounds were first discovered in the 1920s, but, after some interest in the middle of the 20th century, were largely ignored in research. Compared to other diboron compounds, tetrahalodiboranes are fairly unstable and historically have been difficult to prepare; thus, their use in synthetic chemistry is largely unexplored, and research on tetrahalodiboranes has stemmed from fundamental interest in their reactivity. Recently, there has been a resurgence in interest in tetrahalodiboranes, particularly in diboron tetrafluoride as a reagent to promote doping of silicon with for use in semiconductor devices.