
Vitamin K refers to structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation or for controlling binding of calcium in bones and other tissues. The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor. The presence of uncarboxylated proteins indicates a vitamin K deficiency. Carboxylation allows them to bind (chelate) calcium ions, which they cannot do otherwise. Without vitamin K, blood coagulation is seriously impaired, and uncontrolled bleeding occurs. Research suggests that deficiency of vitamin K may also weaken bones, potentially contributing to osteoporosis, and may promote calcification of arteries and other soft tissues.

Methionine is an essential amino acid in humans. As the substrate for other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG.

Homocysteine is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine by the removal of its terminal Cε methyl group. In the body, homocysteine can be recycled into methionine or converted into cysteine with the aid of certain B-vitamins.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The Enzyme commission has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

Trimethylglycine (TMG) is an amino acid derivative that occurs in plants. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because, in the 19th century, it was discovered in sugar beets. Since then, many other betaines have been discovered, and the more specific name glycine betaine distinguishes this one.

Orphenadrine is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs. This substance is considered a dirty drug due to its multiple mechanism of action in different pathways. It was discovered and developed in the 1940s.

Methyl benzoate is an organic compound. It is an ester with the chemical formula C6H5CO2CH3. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.

Menadione is an organic compound with the formula C6H4(CO)2C2H(CH3). It is an analog of 1,4-naphthoquinone with a methyl group in the 2-position. It is occasionally used as a nutritional supplement in animal feed because of its vitamin K activity.
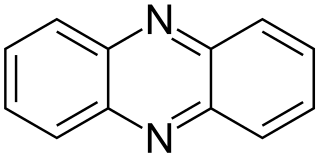
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltrasferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the nucleophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.

Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.

Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Norway and Sweden and is used for severe pain.
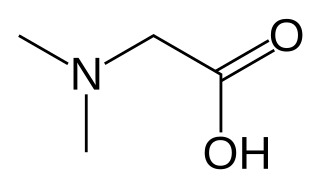
Dimethylglycine (DMG) is a derivative of the amino acid glycine with the structural formula (CH3)2NCH2COOH. It can be found in beans and liver. It can be formed from trimethylglycine upon the loss of one of its methyl groups. It is also a byproduct of the metabolism of choline.

Perzinfotel (EAA-090) is a drug which acts as a potent NMDA antagonist. It has neuroprotective effects and has been investigated for the treatment of stroke, but lacks analgesic effects. Nevertheless, it shows a good safety profile compared to older drugs, although further development of this drug has been discontinued.

Indeloxazine (INN) is an antidepressant and cerebral activator that was marketed in Japan and South Korea by Yamanouchi Pharmaceutical Co., Ltd for the treatment of psychiatric symptoms associated with cerebrovascular diseases, namely depression resulting from stroke, emotional disturbance, and avolition. It was marketed from 1988 to 1998, when it was removed from the market reportedly for lack of effectiveness.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.

N-Methyltyramine (NMT), also known as 4-hydroxy-N-methylphenethylamine, is a human trace amine and natural phenethylamine alkaloid found in a variety of plants. As the name implies, it is the N-methyl analog of tyramine, which is a well-known biogenic trace amine with which NMT shares many pharmacological properties. Biosynthetically, NMT is produced by the N-methylation of tyramine via the action of the enzyme phenylethanolamine N-methyltransferase in humans and tyramine N-methyltransferase in plants.

1,4-Naphthoquinone or para-naphthoquinone is an organic compound derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.

4-Amino-2-methyl-1-naphthol is a menadione analog. Its hydrochloride (HCl) salt is often called vitamin K5. The HCl salt has been used as a medicine for vitamin K deficiency under tradenames such as Synkamin, which was sold by Parke-Davis, but has since been discontinued.

2-Methylnaphthalene-1,4-diamine is a synthetic menadione analog with vitamin K activity.



















