
Hypocalcemia is a medical condition characterized by low calcium levels in the blood serum. The normal range of blood calcium is typically between 2.1–2.6 mmol/L while levels less than 2.1 mmol/L are defined as hypocalcemic. Mildly low levels that develop slowly often have no symptoms. Otherwise symptoms may include numbness, muscle spasms, seizures, confusion, or cardiac arrest.
Hypercalcemia, also spelled hypercalcaemia, is a high calcium (Ca2+) level in the blood serum. The normal range is 2.1–2.6 mmol/L (8.8–10.7 mg/dL, 4.3–5.2 mEq/L), with levels greater than 2.6 mmol/L defined as hypercalcemia. Those with a mild increase that has developed slowly typically have no symptoms. In those with greater levels or rapid onset, symptoms may include abdominal pain, bone pain, confusion, depression, weakness, kidney stones or an abnormal heart rhythm including cardiac arrest.
Disorders of calcium metabolism occur when the body has too little or too much calcium. The serum level of calcium is closely regulated within a fairly limited range in the human body. In a healthy physiology, extracellular calcium levels are maintained within a tight range through the actions of parathyroid hormone, vitamin D and the calcium sensing receptor. Disorders in calcium metabolism can lead to hypocalcemia, decreased plasma levels of calcium or hypercalcemia, elevated plasma calcium levels.
Hypoparathyroidism is decreased function of the parathyroid glands with underproduction of parathyroid hormone (PTH). This can lead to low levels of calcium in the blood, often causing cramping and twitching of muscles or tetany, and several other symptoms. It is a very rare disease. The condition can be inherited, but it is also encountered after thyroid or parathyroid gland surgery, and it can be caused by immune system-related damage as well as a number of rarer causes. The diagnosis is made with blood tests, and other investigations such as genetic testing depending on the results. The primary treatment of hypoparathyroidism is calcium and vitamin D supplementation. Calcium replacement or vitamin D can ameliorate the symptoms but can increase the risk of kidney stones and chronic kidney disease. Additionally, medications such as recombinant human parathyroid hormone or teriparatide may be given by injection to replace the missing hormone.

Hyperparathyroidism is an increase in parathyroid hormone (PTH) levels in the blood. This occurs from a disorder either within the parathyroid glands or as response to external stimuli. Symptoms of hyperparathyroidism are caused by inappropriately normal or elevated blood calcium excreted from the bones and flowing into the blood stream in response to increased production of parathyroid hormone. In healthy people, when blood calcium levels are high, parathyroid hormone levels should be low. With long-standing hyperparathyroidism, the most common symptom is kidney stones. Other symptoms may include bone pain, weakness, depression, confusion, and increased urination. Both primary and secondary may result in osteoporosis.

Parathyroidectomy is the surgical removal of one or more of the (usually) four parathyroid glands. This procedure is used to remove an adenoma or hyperplasia of these glands when they are producing excessive parathyroid hormone (PTH): hyperparathyroidism. The glands are usually four in number and located adjacent to the posterior surface of the thyroid gland, but their exact location is variable. When an elevated PTH level is found, a sestamibi scan or an ultrasound may be performed in order to confirm the presence and location of abnormal parathyroid tissue.

Cinacalcet, sold under the brand name Sensipar among others, is a medication used to treat tertiary hyperparathyroidism, parathyroid carcinoma, and primary hyperparathyroidism. Cinacalcet acts as a calcimimetic by allosteric activation of the calcium-sensing receptor that is expressed in various human organ tissues.
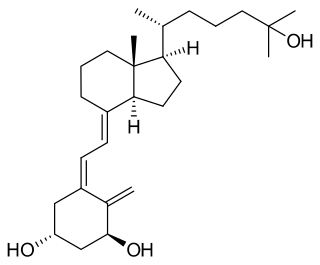
Calcitriol is the active form of vitamin D, normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It is a hormone which binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes. Calcitriol increases blood calcium (Ca2+) mainly by increasing the uptake of calcium from the intestines.
Renal osteodystrophy is currently defined as an alteration of bone morphology in patients with chronic kidney disease (CKD). It is one measure of the skeletal component of the systemic disorder of chronic kidney disease-mineral and bone disorder (CKD-MBD). The term "renal osteodystrophy" was coined in 1943, 60 years after an association was identified between bone disease and kidney failure.

Primary hyperparathyroidism is a medical condition where the parathyroid gland produce excess amounts of parathyroid hormone (PTH). The symptoms of the condition relate to the resulting elevated serum calcium (hypercalcemia), which can cause digestive symptoms, kidney stones, psychiatric abnormalities, and bone disease.
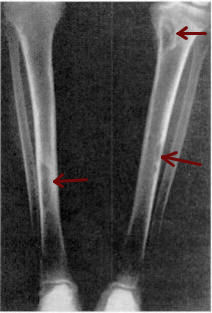
Osteitis fibrosa cystica is a skeletal disorder resulting in a loss of bone mass, a weakening of the bones as their calcified supporting structures are replaced with fibrous tissue, and the formation of cyst-like brown tumors in and around the bone. Osteitis fibrosis cystica (OFC), also known as osteitis fibrosa, osteodystrophia fibrosa, and von Recklinghausen's disease of bone, is caused by hyperparathyroidism, which is a surplus of parathyroid hormone from over-active parathyroid glands. This surplus stimulates the activity of osteoclasts, cells that break down bone, in a process known as osteoclastic bone resorption. The hyperparathyroidism can be triggered by a parathyroid adenoma, hereditary factors, parathyroid carcinoma, or renal osteodystrophy. Osteoclastic bone resorption releases minerals, including calcium, from the bone into the bloodstream, causing both elevated blood calcium levels, and the structural changes which weaken the bone. The symptoms of the disease are the consequences of both the general softening of the bones and the excess calcium in the blood, and include bone fractures, kidney stones, nausea, moth-eaten appearance in the bones, appetite loss, and weight loss.

Secondary hyperparathyroidism is the medical condition of excessive secretion of parathyroid hormone (PTH) by the parathyroid glands in response to hypocalcemia, with resultant hyperplasia of these glands. This disorder is primarily seen in patients with chronic kidney failure. It is sometimes abbreviated "SHPT" in medical literature.

Tertiary hyperparathyroidism is a condition involving the overproduction of the hormone, parathyroid hormone, produced by the parathyroid glands. The parathyroid glands are involved in monitoring and regulating blood calcium levels and respond by either producing or ceasing to produce parathyroid hormone. Anatomically, these glands are located in the neck, para-lateral to the thyroid gland, which does not have any influence in the production of parathyroid hormone. Parathyroid hormone is released by the parathyroid glands in response to low blood calcium circulation. Persistent low levels of circulating calcium are thought to be the catalyst in the progressive development of adenoma, in the parathyroid glands resulting in primary hyperparathyroidism. While primary hyperparathyroidism is the most common form of this condition, secondary and tertiary are thought to result due to chronic kidney disease (CKD). Estimates of CKD prevalence in the global community range from 11 to 13% which translate to a large portion of the global population at risk of developing tertiary hyperparathyroidism. Tertiary hyperparathyroidism was first described in the late 1960s and had been misdiagnosed as primary prior to this. Unlike primary hyperparathyroidism, the tertiary form presents as a progressive stage of resolved secondary hyperparathyroidism with biochemical hallmarks that include elevated calcium ion levels in the blood, hypercalcemia, along with autonomous production of parathyroid hormone and adenoma in all four parathyroid glands. Upon diagnosis treatment of tertiary hyperparathyroidism usually leads to a surgical intervention.

Calciphylaxis, also known as calcific uremic arteriolopathy (CUA) or “Grey Scale”, is a rare syndrome characterized by painful skin lesions. The pathogenesis of calciphylaxis is unclear but believed to involve calcification of the small blood vessels located within the fatty tissue and deeper layers of the skin, blood clots, and eventual death of skin cells due to lack of blood flow. It is seen mostly in people with end-stage kidney disease but can occur in the earlier stages of chronic kidney disease and rarely in people with normally functioning kidneys. Calciphylaxis is a rare but serious disease, believed to affect 1-4% of all dialysis patients. It results in chronic non-healing wounds and indicates poor prognosis, with typical life expectancy of less than one year.
Hypercalciuria is the condition of elevated calcium in the urine. Chronic hypercalciuria may lead to impairment of renal function, nephrocalcinosis, and chronic kidney disease. Patients with hypercalciuria have kidneys that excrete higher levels of calcium than normal, for which there are many possible causes. Calcium may come from one of two paths: through the gut where higher than normal levels of calcium are absorbed by the body or mobilized from stores in the bones. After initial 24 hour urine calcium testing and additional lab testing, a bone density scan (DSX) may be performed to determine if the calcium is being obtained from the bones.

Milk-alkali syndrome (MAS), also referred to as calcium-alkali syndrome, is the third most common cause of hypercalcemia. Milk-alkali syndrome is characterized by elevated blood calcium levels, metabolic alkalosis, and acute kidney injury.
Many conditions are associated with disorders of the function of the parathyroid gland. Some disorders may be purely anatomical resulting in an enlarged gland which will raise concern. Such benign disorders, such as parathyroid cyst, are not discussed here. Parathyroid diseases can be divided into those causing hyperparathyroidism, and those causing hypoparathyroidism.
Calcium acetate/magnesium carbonate is a fixed-dose combination drug that contains 110 mg calcium and 60 mg magnesium ions and is indicated as a phosphate binder for dialysis patients with hyperphosphataemia. It is registered by Fresenius Medical Care under the trade names Renepho (Belgium) and OsvaRen.
Chronic kidney disease–mineral and bone disorder (CKD-MBD) is one of the many complications associated with chronic kidney disease. It represents a systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following:











